Acid catalyzed hydration is a chemical process that happens when an acidic catalyst causes the addition of water to an unsaturated substrate. In an acidic media, for example, an ethene solution can undergo hydration to create alcohol.
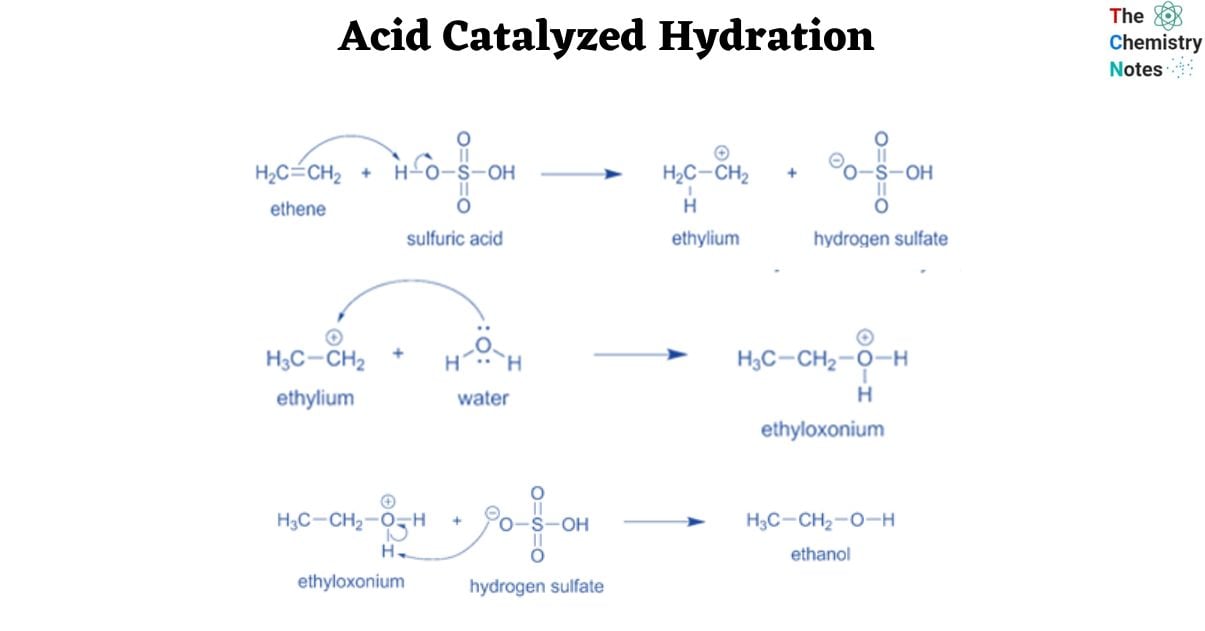
Sulfuric acid and phosphoric acid are two often utilized acidic catalysts for this reaction. Hydronium ions are generated when they are combined with the water reactant. These are the strongest acids that can liquefy in an aqueous solution.
What is Acid Catalyzed Hydration?
An acid catalyzed hydration reaction occurs when an unsaturated compound is converted to a saturated compound in the presence of an acid catalyst. In the presence of an acid catalyst, acid catalyzed hydration processes are extremely effective for dissolving double and triple bonds. Acid catalyzed hydration reactions are very useful at removing double and triple bonds in the presence of an acid catalyst.
This acid-catalyzed hydration reaction takes place in a three-step sequence of reactions.
- The C=C double bond attacks one of the positively charged hydrogen atoms of the hydronium ions in the first phase of the process. This results in the formation of a positively charged intermediate carbocation, which combines with oxygen in water, converting the unsaturated substrate into an oxonium ion.
- In the second stage, an oxygen atom from another water molecule forms a new bond with the carbon, resulting in the formation of the positively charged O atom.
- Water then takes one of the hydrogen atoms from the oxygen, forming a hydroxyl (-OH) group. This regenerates the hydronium ion catalyst for the subsequent reaction cycle.
Example of Acid Catalyzed Hydration
One example of acid catalyzed hydration is the hydration of ethene.
CH₂=CH₂ + H-OH → H-CH₂-CH₂-OH
Sulfuric acid and phosphoric acid are two popular acid catalysts. They react with water to produce hydronium ions, the most powerful acid that can exist in aqueous solution.
There are three steps in the reaction process.
Step 1: In the first step of the reaction, the C=C double bond attacks one of the positively charged hydrogen atoms of the hydronium ions. This leads in the development of a positively charged intermediate carbocation, which interacts with oxygen in water to generate an oxonium ion from the unsaturated substrate.
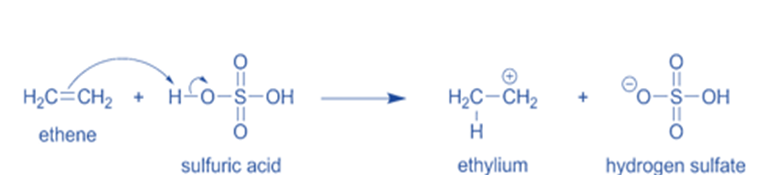
Step 2: The positively charged O atom is formed in the second step when an oxygen atom from a different water molecule makes a new bond with the carbon.
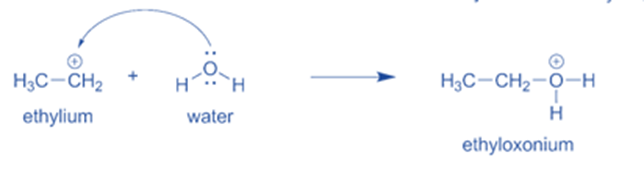
Step 3: Water then forms a hydroxyl (-OH) group by removing one hydrogen atom from the oxygen. This regenerates the hydronium ion catalyst for the next reaction cycle.
Acid Catalyzed Hydration of Alkene
The addition of water to an alkene results in the formation of alcohol by acid-catalyzed hydration:
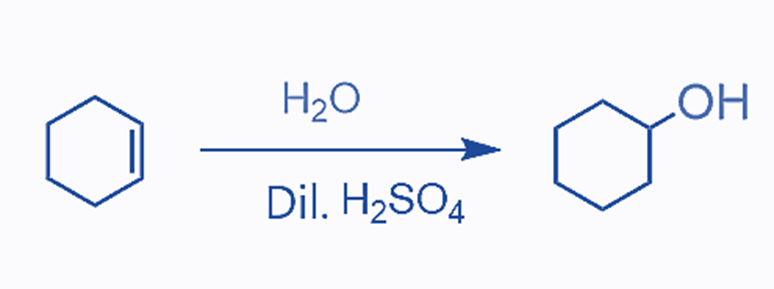
The reaction proceeds in a stepwise manner, beginning with the protonation of the double bond:
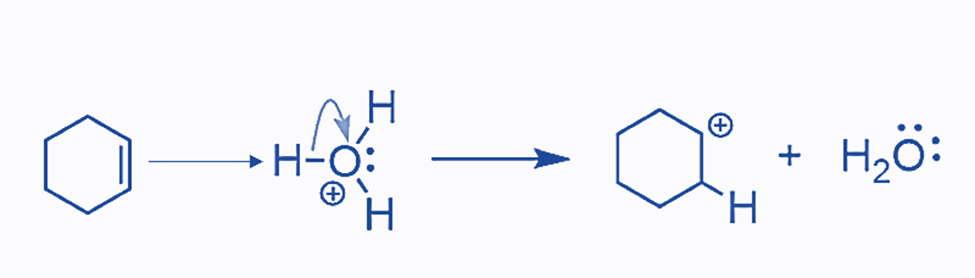
The presence of an acid is required since water is a weak acid and cannot protonate the double bond. However, the hydronium ion generated in acid solutions is powerful enough to attack the electron-rich double bond, resulting in the formation of a carbocation. Water can now attack the carbocation after it has formed:
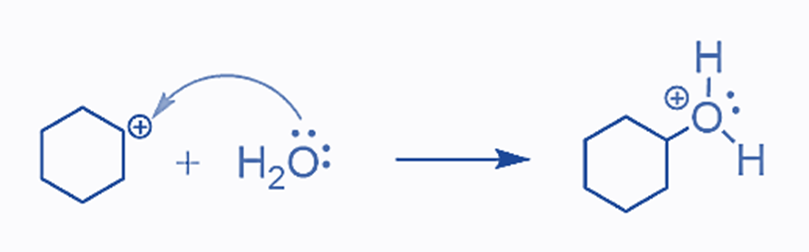
The final product of deprotonation of the hydronium ion is the equivalent alcohol:
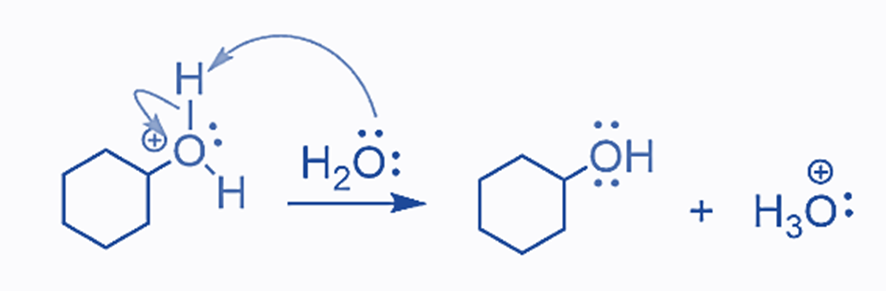
All of the stages in acid-catalyzed alkene hydration are reversible (as are most reactions; it is only a matter of how significant the reaction is).
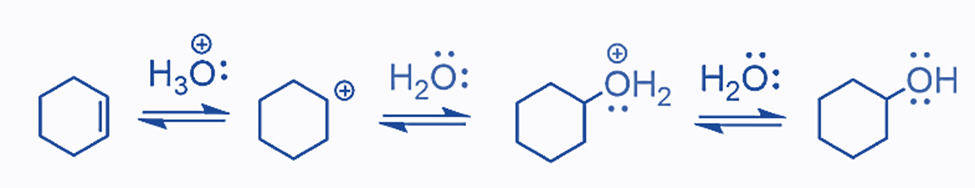
The protonation of the double is the rate-determining step since the alkene and hydronium ion bonds are broken but only one bond is formed.
It’s also important to note that the reaction is catalyzed by a diluted acidic solution because a strong acid would cause the alcohol to be eliminated by E1:

Acid-catalyzed hydration Regio chemistry
The acid-catalyzed hydration of cyclohexene results in the formation of one alcohol as the main product. This is because cyclohexene is a symmetrical alkene with no preference for where the OH group is added. However, if an unsymmetrical alkene is employed, the OH group can be placed in one of two places. According to the Markovnikov’s rule, this process is comparable to the addition of HX acids to alkenes. Remember that for an unsymmetrical alkene, the more stable carbocation defines the reaction’s main product:
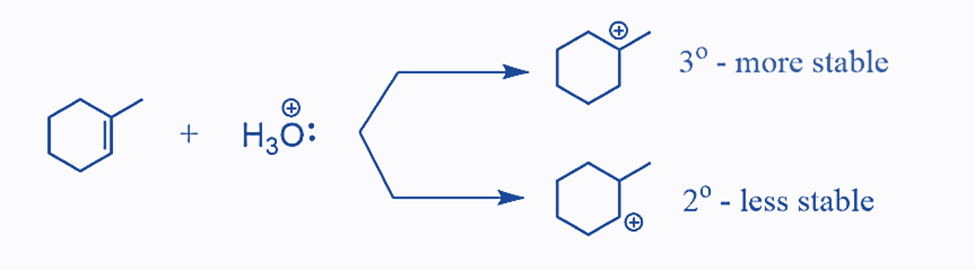
The first carbocation is the more stable intermediate in this case, and it determines the formation of the corresponding alcohol.
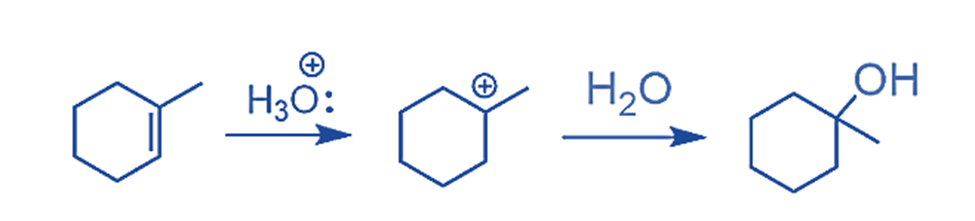
In conclusion, the regioselectivity of acid-catalyzed hydration shows that the OH group preferentially attaches to the more substituted carbon.
References
- https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_Chem_420_-_Organic_Chemistry_I/Text/09%3A_Reactions_of_Alkenes/9.04%3A_Hydration%3A_Acid_Catalyzed_Addition_of_Water
- https://leah4sci.com/acid-catalyzed-hydration-of-alkenes-reaction-and-mechanism/
- https://socratic.org/organic-chemistry-1/alkene-and-alkyne-addition-reactions/acid-catalyzed-hydration
- https://www.jove.com/science-education/11776/acid-catalyzed-hydration-of-alkenes-to-alcohols
- https://askfilo.com/user-question-answers-chemistry/what-is-acid-catalyzed-hydration-35363432353132
- https://www.chemistrysteps.com/acid-catalyzed-hydration-of-alkenes-practice-problems/
