Acetone is a colorless liquid ketone that is volatile and combustible. It is the simplest and most basic aliphatic ketone, with the formula CH3COCH3. It is also known as 2-propanone, or dimethyl ketone ad acts as an organic solvent for various industrial and chemical applications.
Acetone has a strong, floral, or unpleasant odor and is miscible with water, ether, and ethanol. Acetone can be found in car exhaust, plants, trees, and forest fires. It is also found in the human body’s urine and blood. It is naturally formed as a byproduct of the breakdown of acetoacetic acid. It is also commercially produced as a precursor to methyl methacrylate. Acetone was initially produced by alchemists and is manufactured through the dry distillation of metal acetates.
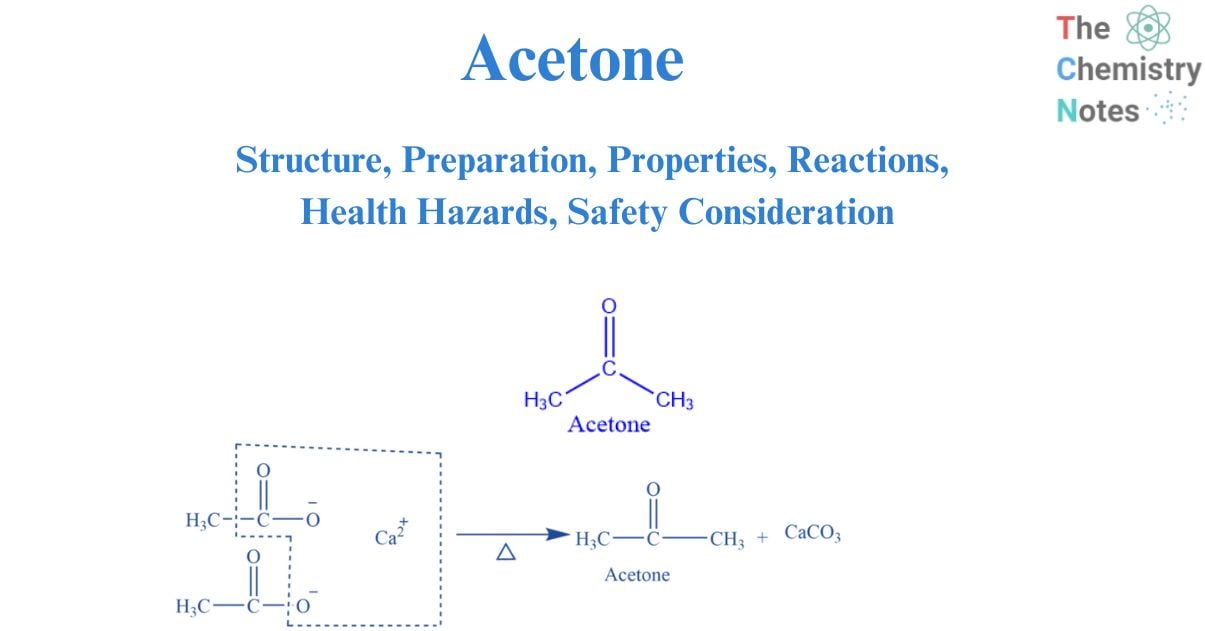
Structure of acetone
The carbonyl group, which consists of a carbon atom double-bonded to an oxygen atom, is the functional group that makes up acetone. Functional groups are used to classify organic molecules; substances with solely the carbonyl functional group are categorized as ketones. As the central carbon has been sp2 hybridized, the bond angles between the carbon atoms are predicted to be 120. The geometry of acetone is trigonal planar. It is mostly utilized in medicine and cosmetics. It is widely used as an antiseptic and solvent.
Preparation of acetone
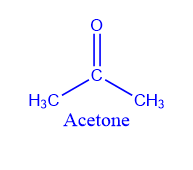
Catalytic decomposition of the acid:
Vapors of ethanoic acid on passing over MnO or ThO2 produce Acetone.
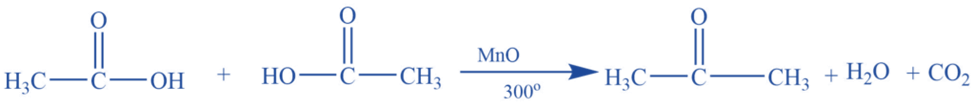
By dry distillation of calcium acetate (Laboratory preparation)
Acetone is produced in a laboratory by dry distilling anhydrous calcium acetate.
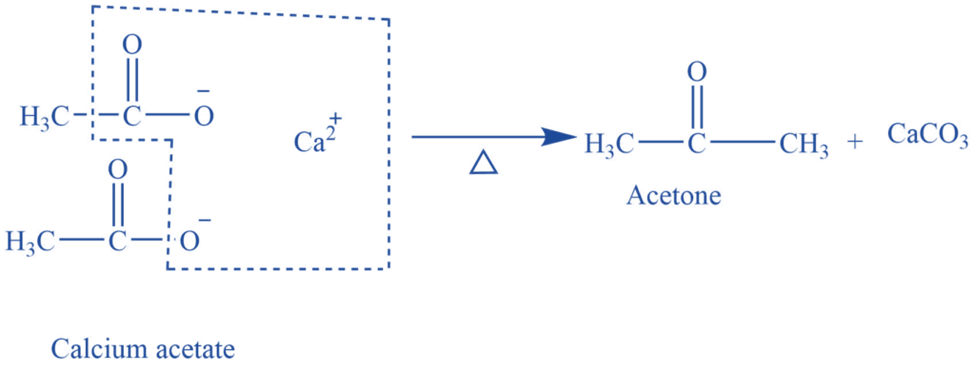
In a glass retort equipped with a condenser and receiver, 30-40 grams of calcium acetate and an equal amount of sodium acetate are heated. Acetone vapors are formed and condensed to liquid acetone by passing through a condenser. The receiver collects acetone. Thus produced acetone is not pure and is purified by shaking it with a saturated sodium bisulfite solution, which is followed by the separation of crystals of acetone sodium bisulfite salt. To obtain pure acetone, the crystal is first cleaned, heated with sodium carbonate, dried on anhydrous calcium chloride, and then distilled at a temperature of 56 o C.
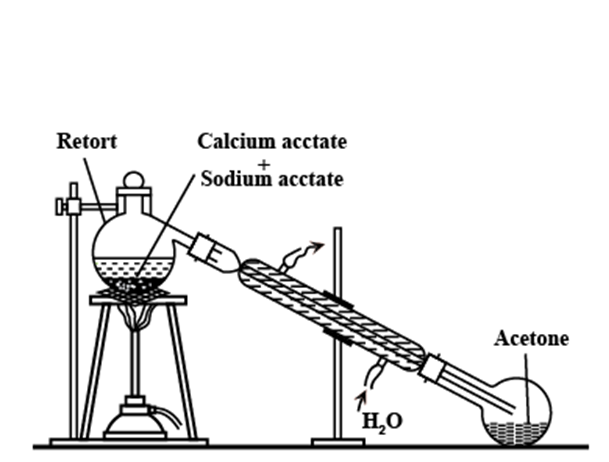
Fig: Laboratory preparation of acetone
image source: https://www.toppr.com/ask/question/describe-the-method-for-the-preparation-of-acetone-in-laboratory/
From gem dihalides
Acetone is produced by alkaline hydrolysis of gem-dihalides bearing two halogen groups at the nonterminal hydrogen.

From pyroligneous acid
Pyroligneous acid, which contains acetic acid, acetone, and methyl alcohol, is distilled in a copper vessel and the vapors are passed through heated lime milk. Acetic acid reacts with calcium acetate to generate nonvolatile calcium acetate. Unabsorbed methanol and acetone vapors are condensed and fractionally distilled. Acetone distills at a temperature of 56oC.
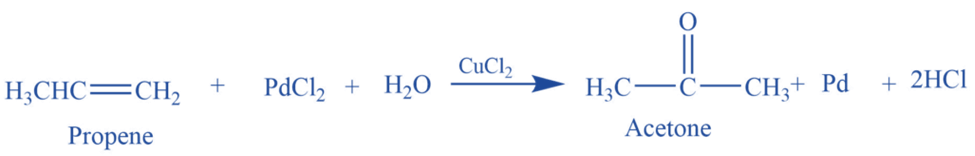
From Wacker’s process
Propene reacts with an acidified aqueous solution of palladium chloride and cupric chloride to produce Acetone.
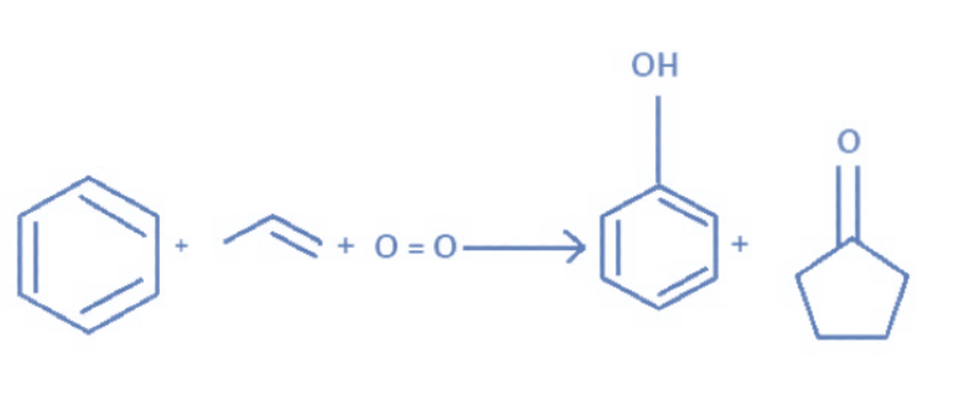
From cumene process (industrial process)
The cumene process produces 83% of acetone in the industry. Benzene is alkylated with propylene to form cumene, which is then oxidized by air to yield phenol and acetone. Propene is transformed directly or indirectly in this way to produce acetone.
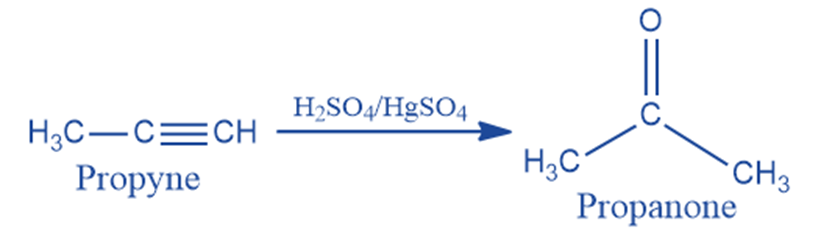
From alkyne
Propyne is hydrated in the presence of dilute sulphuric acid and mercuric sulfate to produce acetone.
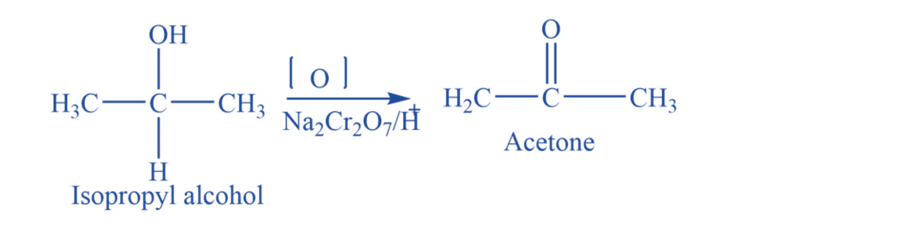
From alcohol
Isopropyl alcohol undergoes an oxidation reaction in the presence of different oxidizing agents like Na2Cr2O7+ H2SO4, or K2Cr2O7 to produce acetone.
Properties of acetone
- It is a colorless liquid with a pungent or irritating odor.
- It is a flammable liquid with a boiling point of 58oC.
- The density of acetone is 0.7845 g/cm3 (25°C)
- It is completely miscible with water, alcohol, and ether.
Reactions of acetone
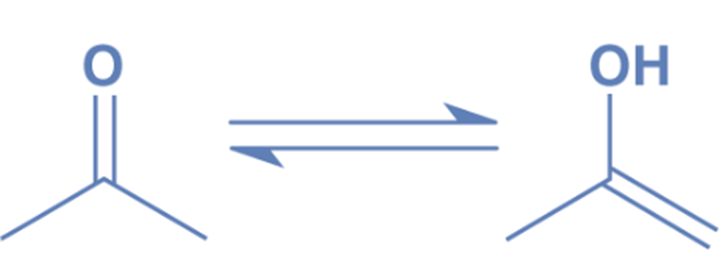
Keto-enol tautomerism
Like other ketones, acetone exhibits keto-enol tautomerism, which means that the enol isomer (CH3)C(OH)=(CH2) is in equilibrium with the nominal keto structure (CH3)2C=O.
At room temperature, just 2.4107% of the molecules in acetone vapor are enols. The enol form is essential in a number of chemical processes.
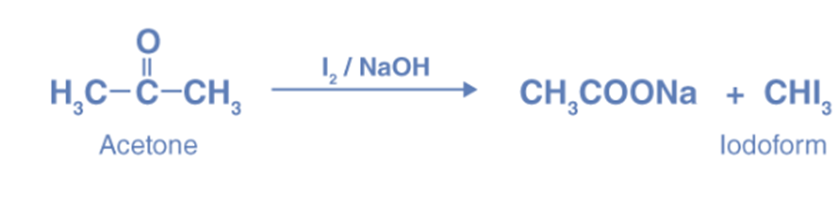
Haloform reaction
The Haloform reaction produces haloforms (CH3X) by completely halogenating acetone (CH3COCH3), acetophenone (PhCOCH3), or acetaldehyde (HCOCH3) in the presence of a base. Acetyl groups can be converted into carboxyl groups through this process, and it can also create cyanide, chloroform (CHCl3), bromoform (CHBr3), or iodoform (CHI3).
The reaction of methyl ketones with halogen in a basic solution produces a polyhalogenated product due to the substitution of three alpha-hydrogens in the methyl group by the halogen group. Once these hydrogens are replaced by halogen, the other hydrogen atoms in that carbon become more acidic, causing additional substitution.
Due to the presence of the CH3-C=O group, acetone undergoes the haloform reaction, where it reacts with halogen in the presence of alkali to form haloform and acid salt.
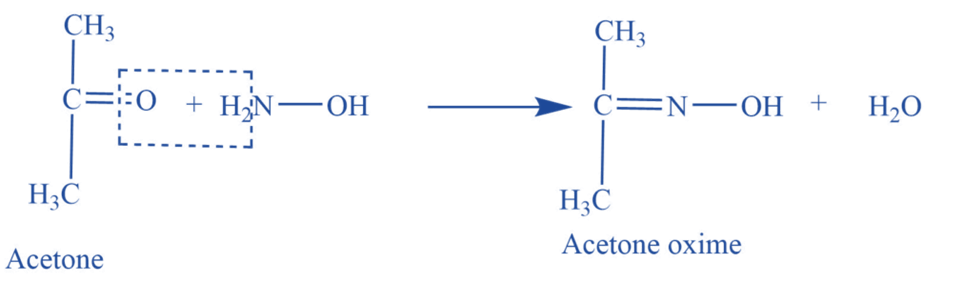
Reaction with hydroxylamine
Acetone reacts with hydroxylamine (NH2OH) to give acetone oxime.
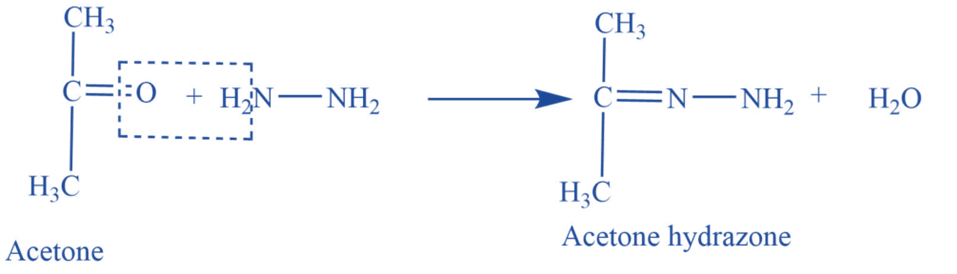
Reaction with hydrazine
Acetone reacts with hydrazine (NH2-NH2) to produce acetone hydrazine.
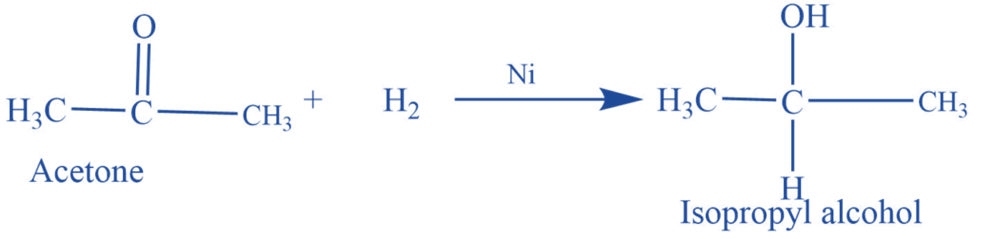
Reduction reaction
Acetone undergoes a reduction reaction in the presence of hydrogen and Ni or Pt catalyst to produce isopropyl alcohols.
Wolf – Kishner reaction
Acetone reacts with a basic solution of hydrazine to form propane.

Uses of acetone
- It’s utilized in medicines as a chemical intermediary and as a solvent for vinyl and acrylic resins, lacquers, alkyd paints, inks, cosmetics (including nail polish remover), and varnishes.
- Acetone is widely utilized in the production of synthetic fibers.
- It is also used as a starting material in the synthesis of a wide range of chemicals.
- It is used for acne treatment.
- It is used to transport the highly combustible chemical fuel acetylene safely.
- In the petroleum industry, it is added to natural gas fuel to improve efficiency.
- It is used in the electronics industry to clean small electrical devices.
- Low-grade acetone is commonly used in academic laboratories to clean glassware.
- It is used to remove grease and gum from textiles like wool and silk.
- It is used in the production of lacquers for automobiles and furniture.
- Acetone is an excellent solvent. It is used to dissolve washing, cellulose, artificial silk, cordite, and other materials.
- It is also utilized in the production of synthetic scents and rubber.
Risks of Using Acetone
- It is highly flammable. acetone liquid and vapor catch fire easily.
- Acetone is an irritant, which means it can cause skin irritation. Acetone fumes can irritate the eyes, nose, throat, and lungs if they are inhaled.
- It can also induce coughing, dizziness, and headache.
- Acetone poisoning causes slurred speech, slow breathing, loss of physical coordination, and unconsciousness.
Working with Acetone: Safety Considerations
- Acetone should only be used in well-ventilated areas.
- Avoid direct skin contact.
- Put on gloves and goggles while working with acetone.
- keep open flames and cigarettes away from acetone.
- Always keep bottle lids tightly closed when not in use, and dispose of any acetone-soaked cotton wool in a receptacle with a tight-fitting cover to keep fumes from escaping.
- When you are no longer using the product, thoroughly wash your hands before eating, drinking, or touching your face.
References
- https://www.vedantu.com/chemistry/uses-of-acetone.
- https://byjus.com/chemistry/acetone/
- https://www.britannica.com/science/ketone.
- https://chemistrypage.in/acetone-formula-and-structure/.
- https://infinitylearn.com/surge/chemistry/uses-of-acetone/
