The gas laws are a set of laws that explain how gases behave by showing relationships between the following:
- The volume occupied by the gas.
- The pressure of gases
- The absolute temperature of the gas.
- The amount of a gaseous substance
Gas Laws to be studied are listed below:
Boyle’s Law
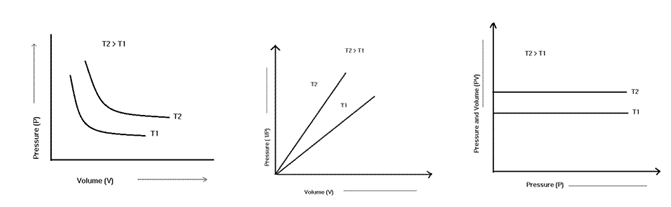
Boyle’s law states that when temperature and concentration are kept constant, the pressure exerted by a gas is inversely proportional to the volume occupied by it.
When the temperature remains constant,
Boyle’s Law states that P1V1 = P2V2
Boyle’s Law also says that P V = k, where k is the proportionality constant.
Boyle’s Law Formula
The relationship between volume and pressure (at constant mass and temperature) can be expressed mathematically as follows:
V ∝ (1/P) When T is Constant
or, V = K. 1/P
When P is the pressure exerted by a gas, V is the volume it occupies, and K is the proportionality constant whose value depends on the gas’s mass and temperature, Rearranging the equation gives,
P * V = K ……………(1)
Thus, the product of pressure and volume for a given gas is always constant at a constant temperature. If V1 is the volume of a given mass of a gas at pressure P1 and V2 is the volume of the gas when pressure is changed to P2, then equation (1) follows:
P1V1= K = P2V2 when T remains constant
or, P1/P2 = V1/V2 ..…………..(2)
where,
P1 is the initial pressure exerted by the gas
V1 is the initial volume occupied by the gas
P2 is the final pressure exerted by the gas
V2 is the final volume occupied by the gas
The mathematical expression for Boyle’s law formula is P₁V₁ = P₂V₂.
Charle’s Law
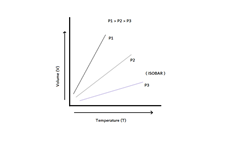
The volume of the given mass of gas expands or contracts by 1th/273 of its original volume for every 1 degree C rise or fall in temperature when pressure is constant.
In other words, when the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be in direct proportion.
According to Charles’s law, the directly proportional relationship between temperature and volume is mathematically as follows:
V ∝ T
Or
VT=k
Where,
V is the volume of the given gas.
T is the temperature of the given gas, measured in kelvins.
k is a constant obtained by dividing V by T.
Gay-Lussac’s Law
Gay-Lussac’s Law, sometimes known as the law of combining volumes, is a fundamental principle in the field of chemistry.
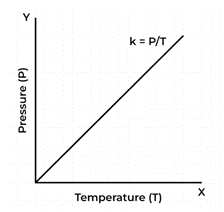
According to Gay-Lussac’s Law, a gas’s pressure and temperature are both inversely correlated when kept constant volume and a constant number of moles.
According to Gay-Lussac’s Law, temperature changes in response to changes in pressure.
Several mathematical representations exist for Gay-Lussac’s Law mentioned below:
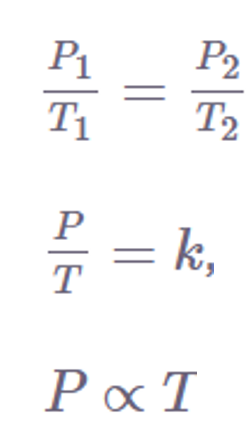
Where:
P is the pressure exerted by the gas
T is the absolute temperature of the gas
k is a constant.
Gay-Lussac’s Formula
Gay-Lussac’s law establishes a mathematical expression that describes the relationship between pressure and temperature, under the condition that volume and mass/moles remain constant. This can be interpreted as:
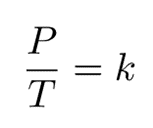
Since they are both equal to the same constant number, we can also relate pressure and temperature at two different points.
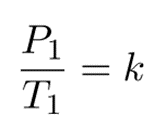
And
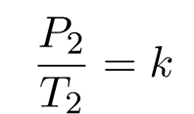
Therefore,
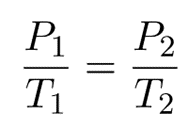
The formula may be observed in various ways.
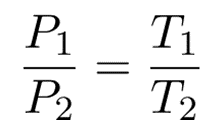
The value of k in these equations can also be determined by rearranging the ideal gas law.
We are maintaining constant volume (V) and moles (n). The entire right-hand side of the lower equation represents a constant value.
Avogadro’s Law
Amedeo Avogadro, an Italian chemist, and scientist, proposed Avogadro’s Law, which states that equal volumes of all gases under identical temperature and pressure circumstances contain an equal number of molecules.
If two distinct gases with the same temperature and pressure occupy the same volume, their number of molecules will be the same. We get the same number of moles of gases if we divide their number of molecules by a set number, (6.023 * 1023), which is the Avogadro number.
number of molecules in gas A = number of molecules in gas B (if V, P, and T are the same).
Where,
V is the Volume of gas
P is the Pressure of gas, and
T is Temperature
From the mole concept
Number of moles in A = N/Avogadro Number
The number of moles in B = N/Avogadro Number.
We know that the Avogadro number (6.023 1023) is constant.
As a result, the number of moles in A = the number of moles in B = n (assumed).
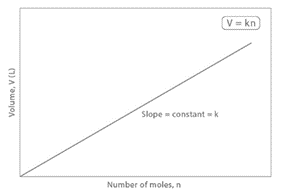
This means that if the volume of gases remains constant under constant temperature and pressure, the number of moles of gases remains constant. It has been discovered experimentally that any gas at 273K and one atmospheric pressure, i.e., at NTP, has a volume of 22.414 liters (or 22.4 liters). When the volume of a gas at NTP is increased, so is the number of moles of the gas.
As a result, at any fixed temperature and pressure, the volume of a gas is proportional to the amount ‘n’, which is the number of moles in the gas. This is known as Avogadro’s Law.
Combined Gas Law
The Combined Gas Law states that when the amount of gas is fixed, the product of pressure (P), volume (V), and temperature (T) is equal to a constant (k).
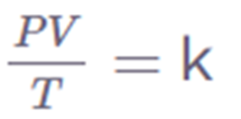
The combined gas law is formed by combining four different laws.
- Charles’ law states that the volume of a gas is directly proportional to its temperature, assuming the pressure and amount of gas remain constant.
- Gay-Lussac’s law is a scientific principle that describes the relationship between the temperature and pressure of a gas.
- Avogadro’s law refers to the relationship between the volume of a gas and the number of particles it contains.
- Boyle’s law is a principle in physics that describes the relationship between the pressure and volume of a gas.
These laws relate one thermodynamic variable to another while keeping all other variables constant. The relationship between these variables is shown by the combined gas law. It states that the product of a system’s pressure, volume, and temperature remains constant.
The Combined Gas Law is written as:
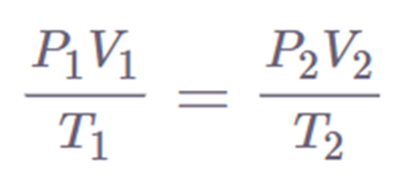
Combined gas law can be mathematically expressed as
k = PV/T
Where,
P = pressure
T = temperature in kelvin
V = volume
K = constant
In two different conditions, the law can be stated as,
Pi Vi/ Ti = Pf Vf / Tf
Where,
Pi = initial pressure
Vi = initial volume
Ti = initial temperature
Pf = final pressure
Vf= final volume
Tf = final temperature
Video on Gas Laws
References
- https://www.chem.fsu.edu/chemlab/chm1045/gas_laws.html
- https://byjus.com/jee/gas-laws/
- https://www.toppr.com/guides/chemistry/states-of-matter/gas-laws/
- https://openstax.org/books/chemistry-2e/pages/9-2-relating-pressure-volume-amount-and-temperature-the-ideal-gas-law
- https://www.ncbi.nlm.nih.gov/books/NBK546592/

