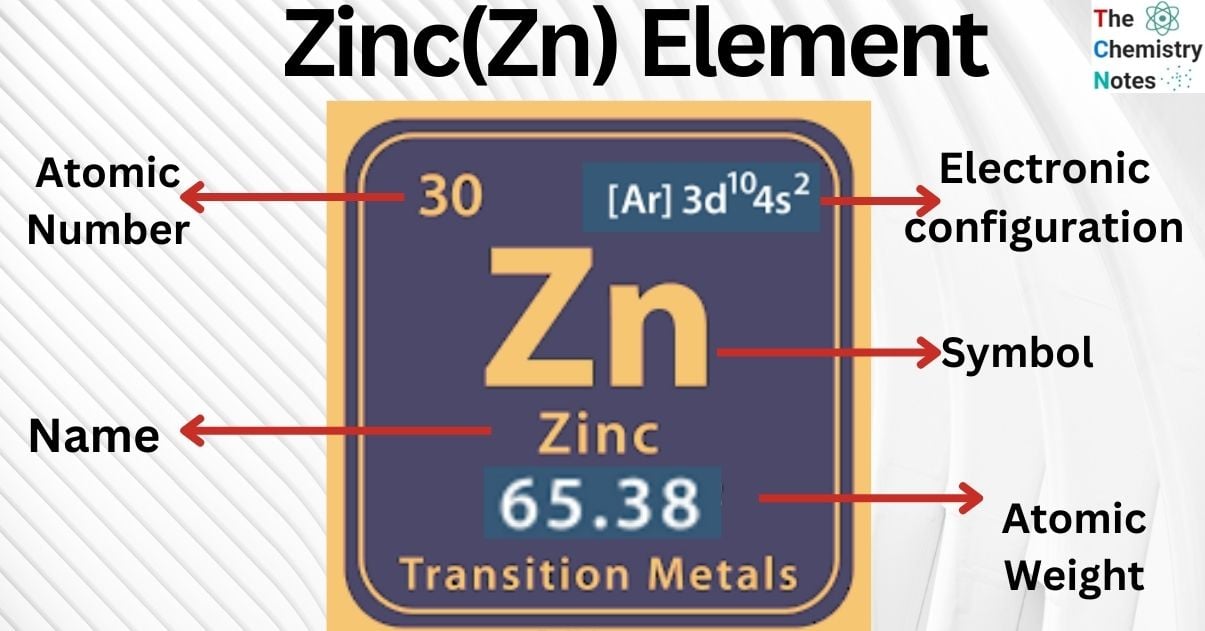
Zinc is the metallic element with the atomic number 30 and is represented by the symbol ‘Zn’ in the periodic table. It belongs to the d-block of group 12 (II) of the periodic table. Zinc has chemical similarity to that of magnesium. It is transition metal and is a bluish-white, glossy, diamagnetic metal; nonetheless, all of its industrial grades have a dull appearance.
Zinc is 23rd most abundant element in Earth’s crust which is little more abundant than copper and and fourth as a percentage of the output of all metals worldwide, behind copper, aluminum, and iron. After iron, it is the second most prevalent trace metal naturally detected in the human body. The main ore mineral in the world is and always has been sphalerite or zinc sulfide.
History of Zinc
- Zinc compounds have been used to create brass for more than 2,500 years, but zinc wasn’t recognized as a distinct element until much later.
- The Charaka Samhita (a sanskrit text on ayurveda), which is thought to have been written between 300 and 500 AD, mentions a metal that, upon oxidation, generates pushpanjan. This material is thought to be zinc oxide.
- Since the Mauryan era (between 322 and 187 BCE), India has had zinc mines operating at Zawar, close to Udaipur. However, it seems that the smelting of metallic zinc here first started around the 12th century AD.
- The tablets were discovered on board the Roman ship Relitto del Pozzino, which sank in 140 BC, and were meant to treat sore eyes. Smithsonite and hydrozincite were two zinc carbonates, used to make the earliest tablets.
- In the medical Lexicon attributed to the Hindu monarch Madanapala (of the Taka dynasty) and published in the year 1374, zinc has been identified as an element with the names Yasada or Jasada.
- The first time metallic zinc was recovered from zinc oxide was in the year 1668 by a Flemish metallurgist.However, Europe claimed that Andreas Marggraf, a German chemist, made the discovery of zinc in 1746.
- The term zinc is derived from the German word “Zinke” which means pointed or spikey.
Occurrence of Zinc
Natural zinc comprises roughly 0.0076% of the Earth’s crust which makes it the 23rd most abundant metal on Earth. Zinc doesn’t exist in its pure form in nature. It is typically found in different kinds of minerals which include:
Zincite: One of the primary minerals in zinc ore deposits is a rare zinc oxide mineral known as zincite (ZnO). It is usually found alongside other zinc minerals like sphalerite and is red or orange in color.
Smithsonite: When primary zinc sulfide minerals such as sphalerite weather and oxidize, the secondary zinc carbonate mineral smithsonite (ZnCo3) is formed. It can typically be found in oxidized zinc ore deposits and may occur in a variety of colors, like white, green, blue, pink, and gray.
Sphalerite: The most important and commonly occurring zinc ore mineral is sphalerite (ZnS). It is usually found in hydrothermal veins, as well as in sedimentary, metamorphic, and igneous rocks. It may vary in color depending on its impurities. It usually has a yellow, green, black, brown, or transparent look.
Hemimorphite: Secondary zinc silicate minerals that occur in zinc ore deposit is hemimorphite (Zn4Si2O7(OH)2H2O). It can usually be found in zinc ore deposits that are oxidized and occur in a variety of colors like white, blue, green, brown, or even colorless.
Franklinite: (ZnFe2O4)
Willemite: (Zn2SiO4)
Zinc ores are found in many countries across different continents. Zinc is the fourth most common metal in use, trailing only iron, aluminum, and copper with an annual production of about 13 million metric tons. About 70% of the world’s zinc originates from mining, while the remaining 30% comes from recycling secondary zinc. Zinc mines are scattered throughout the world, with the main areas being China, Australia, and Peru.
Isotopes Of Zinc
There are five stable isotopes of Zinc (Zn) that are present in nature: 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn.
Naturally occuring zinc isotopes are:
| Isotope | Natural abundance (atom %) |
|---|---|
| 64Zn | 48.63 (60) |
| 66Zn | 27.90 (27) |
| 67Zn | 4.10 (13) |
| 68Zn | 18.75 (51) |
| 70Zn | 0.62 (3) |
Elemental Properties of Zinc
| Electronic Configuration | [Ar] 3d10 4s2 |
| Atomic Number | 30 |
| Atomic Weight | 65.38 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 12, 4, d-block |
| Density | 7.134 g.cm -3 at 20 °C |
| Covalent radius | 122(4) pm |
| Van der Waals radius | 139 pm |
| Electron shells | 2, 8, 18, 2 |
| Electrons | 30 |
| Protons | 30 |
| Neutrons in most abundant isotope | 34 |
Physical Properties of Zinc
- Zinc has an atomic number of 30 and is a bluish-white, lustrous metal.It has a melting point of 1419.58 °C (787.24 °F) and a boiling point of 907 °C (1664 °F).
- The density of Zinc is 7.10 grams per cubic centimeter.
- When freshly shattered, it has a resinous to adamantine shine, but after being exposed to air, it can lose some of its shine.
- Zinc remains brittle and in a crystalline condition at ambient temperature. But when heated between 110°C to 150°C it becomes ductile (it can be pulled out into thin wires) and malleable to some extent (it can be pressed into thin sheets).
- Zinc is a diamagnetic metal, which means it doesn’t possess magnetic properties.
- Zinc serves as a good electrical conductor. Because electrons in nickel are free to move around they are able to carry electrical charge from one end to other.
- Zinc is an excellent thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Zinc is widely used to coat steel and other metals because of its exceptional corrosion resistance. This makes it possible for the zinc’s outermost layer to combine with atmospheric oxygen to form zinc oxide, which offers protection.
- Zinc is odorless and tasteless.
| Color/physical appearance | Bluish silver |
| Melting point/freezing point | 419.527°C, 787.149°F, 692.677 K |
| Boiling point | 907°C, 1665°F, 1180 K |
| Density | 7.134 g cm-3 at 20°C |
| Malleability | No [zinc gains malleability above 100oC] |
| Ductility | No |
Chemical Properties of Zinc
- Reaction of Zinc with Water
Zinc metal does not react with water under normal conditions.
- Reaction of Zinc with Air
Moist air causes zinc metal to tarnish. When zinc metal burns in air, it produces white zinc(II) oxide, which turns yellow when heated for an extended period of time.
2Zn(s) + O2(g) → 2ZnO(s) [white]
- Reaction of Zinc with the Halogens
When zinc (Zn) reacts to either bromine (Br2) or iodine (I2), the compounds zinc(II) bromide, ZnBr2, and zinc iodide, NiI2, are created.
Zn(s) + Br2(g) → ZnBr2(s) [white]
Zn(s) + I2(g) → ZnI2(s) [white]
- Reaction of Zinc with Acids
Zinc metal slowly breaks in weak sulfuric acid to produce solutions that contain the aquated Zn(II) ion and hydrogen gas, H2. In practice, the complex ion [Zn(OH2)6]2+ represents the Zn(II).
Zn(s) + H2SO4(aq) → Zn2+(aq) + SO42-(aq) + H2(g)
Uses Of Zinc
Currently after iron, aluminum, and copper, zinc is the fourth most used metal globally. It has been used for centuries in different types of applications. There are many uses for zinc, but only a handful are covered below.
Used For Galvanization: Steel and iron are typically galvanized using zinc. When exposed to air, a thin layer of zinc oxide forms, ultimately aiding in the prevention of metal corrosion (galvanization), which stops steel or iron from rusting. It is widely utilized in the construction, automotive, and other industries because it provides advantages like longevity, durability, and cost-effectiveness.
Used In Batteries: Zinc batteries are safer and don’t pose the risk of a thermal runaway because they use water-based chemistry, which makes them unable to sustain fire.It is used to make the zinc-carbon and zinc-nickel batteries that are frequently used in toys, flashlights, household appliances, and other items.Compared to lithium batteries, zinc-ion batteries are less expensive, more durable, and safer.
Used As Alloy: Zinc is a weak and brittle metal on its own, and it is used in coating and galvanizing, whereas other metals bear heavy duties. However, when zinc is alloyed with other strong materials, it increases the strength of the alloy. Brass is one of the best alloys of zinc; it contains 55 percent zinc and 95 percent copper. This metal is used for different mechanical uses, jewelry, and art casting. It is also used in statues, musical instruments, and engine parts.Some other examples of zinc alloys are Zamak, Tomac, etc.
Zinc alloys are used in medical, marine and automotive sectors.
Health Effects Of Zinc
- Zinc is a trace element it is present in a human body in a trace amount.Human body doesn’t store excess zinc so it must be obtained from diets.
- Our body needs zinc for immune function, thyroid function, wound healing, blood clotting, and much more. Fish, poultry, and red meat are all common sources of zinc, a vital essential element.
- Although zinc is not seriously harmful for human,excessive zinc consumption can still result in serious health issues like anemia, nausea, vomiting, and irritation of the skin.
- Extremely high zinc concentrations can harm the pancreas, disrupt protein metabolism, and lead to arteriosclerosis. The prolonged usage of zinc chloride can lead to respiratory problems.
Environmental Effects of Zinc
- The output of zinc worldwide is still increasing. Basically, it indicates that more zinc is released into the environment. Because manufacturing facility wastewater contains significant amounts of zinc, it contaminates water.
- Soils contain significant amounts of zinc. When agriculture soils are contaminated with zinc, animals absorb toxic levels.
Watch out this interesting video.
References
- https://www.britannica.com/science/zinc
- Burgess, John (1978). Metal ions in solution. New York: Ellis Horwood. ISBN 978-0-470-26293-1.
- Brady, James E.; Humiston, Gerard E.; Heikkinen, Henry (1983). General Chemistry: Principles and Structure (3rd ed.). John Wiley & Son ISBN 978-0-471-86739-5.
- Ryu M-S, Aydemir TB. Zinc. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Cambridge, Massachusetts: Wiley-Blackwell; 2020:393-408.
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons, Inc. ISBN 978-0-471-19957-1.
- Hinds, John Iredelle Dillard (1908). Inorganic Chemistry: With the Elements of Physical and Theoretical Chemistry (2nd ed.). New York: John Wiley & Sons.
- King JC, Cousins RJ. Zinc. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:189-205.

