The Zeroth Law of Thermodynamics, the First Law of Thermodynamics, the Second Law of Thermodynamics, and the Third Law of Thermodynamics are the four laws of thermodynamics. The zeroth law of thermodynamics was created after the first three, which is an important distinction. However, there was considerable debate over whether it should be known as the fourth law or by another name. The problem arose because the new law effectively replaced the previous three statutes and gave a clearer definition of temperature. Ralph H. Fowler devised the zeroth law. The Zeroth law of thermodynamics frames the idea of temperature as a sign of thermal equilibrium.
What is Thermal Equilibrium?
When a higher temperature object comes into contact with a lower temperature object, heat is transferred to the lower temperature object. In the absence of loss to other objects, the objects will then maintain a constant temperature as they get closer to the same temperature. The state is then referred to as thermal equilibrium.
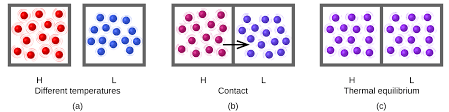
Temperature can be defined as the energy of molecules in a substance. The fast-moving molecules of one material will collide with the slow-moving molecules of another when two objects at different temperatures are kept together. Heat energy will spread until both objects are at the same temperature as a result.
A system is said to have minimized its thermodynamic potential if it is in thermodynamic equilibrium. The total amount of useful work that can be extracted from a system is measured by Helmholtz free energy.
The formula for the equation is A = U – TS;
where,
Helmholtz free energy is A;
The internal energy is U;
The temperature is T, and
S stands for entropy.
For Example: The systems are thus in thermal equilibrium if they do not transfer heat, even if they are capable of doing so due to external factors. The air in the refrigerator and the fruits, for example, are in thermal equilibrium when fruits are stored in the refrigerator overnight. As a result, heat no longer transfers from one source (the fruits) to the other source (the air) or back. However, if the fruits are left outside, heat will transfer from the outside air to the fruits.
Zeroth Law of Thermodynamics
Statement
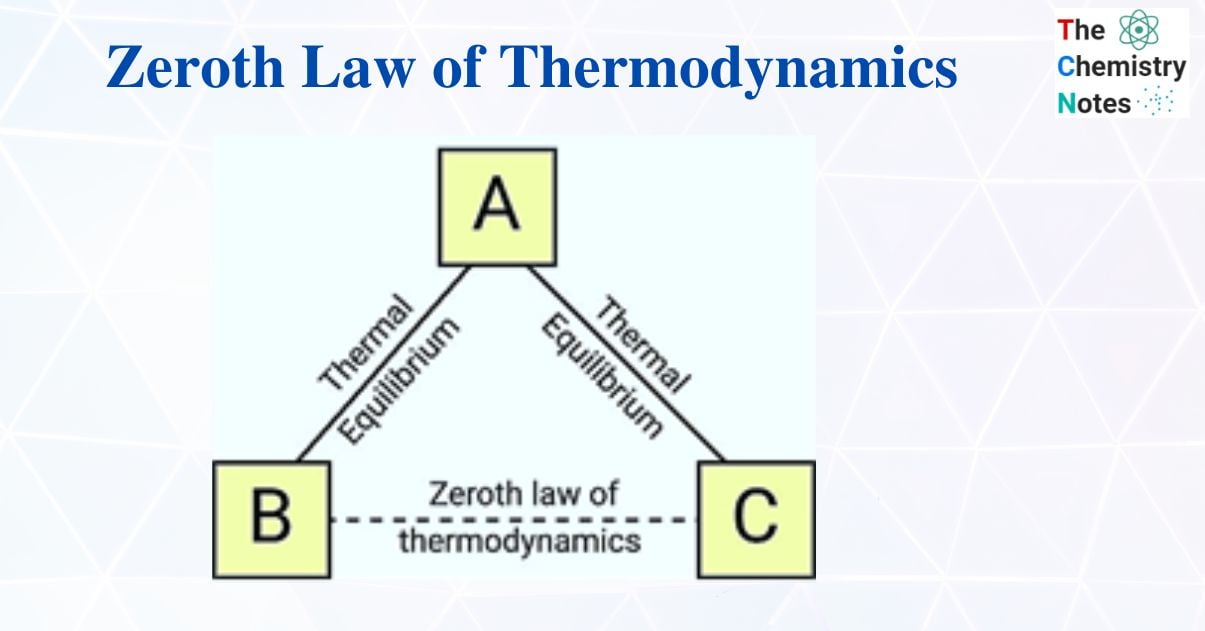
According to the zeroth law of thermodynamics, if two systems are in thermal equilibrium with one another and with a third system, then the first two systems are also in thermal equilibrium with one another. The ability to define a temperature scale and use thermometers as the “third system” is made possible by this property.
Consider the following three systems: A, B, and C. Let thermal equilibrium exist between systems A and C. Systems A and B are in thermal equilibrium when they are considered separately, and the zeroth law predicts that systems B and C will also be in thermal equilibrium.
Explanation
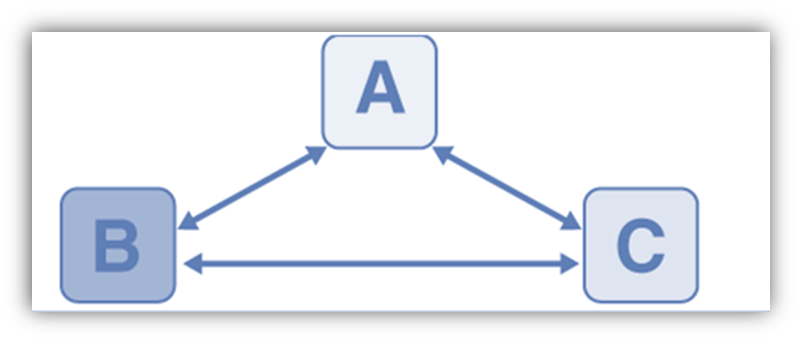
By taking into account three systems, consider two systems A and B that are in contact with one another through a conducting wall and are separated from one another by an adiabatic wall in order to comprehend this law. An adiabatic wall is used to divide systems A and B, but system C is allowed to communicate with both of them. When thermal equilibrium is reached, we wait With C, A, B, and each other are in thermal equilibrium.
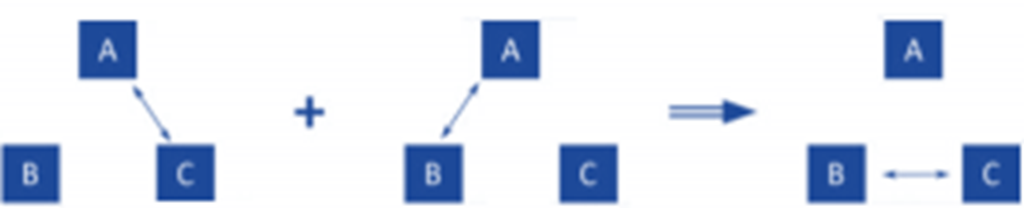
- Numerous experiments have shown that there won’t be any net energy flow between A and B. This serves as experimental support for the proposition that follows.
- Two systems are in thermal equilibrium with one another if they are also both in thermal equilibrium with a third body. The zeroth law of thermodynamics is understood to be this assertion.
- Since temperature shows whether heat will transfer between objects, it is important to measure it, according to the zeroth law of thermodynamics. Regardless of how the objects interact, this will be accurate. Heat can still travel between two objects even if they are not in contact, such as through radiation.
Although it is not explicitly stated, it is implied that temperature exists. All systems in thermal equilibrium with one another will always have the same amount of temperature. The thermal equilibrium between the systems is depicted by the double arrow. Systems B and C are in equilibrium if systems A and C are also in equilibrium, as well as if systems A and B. The temperature in systems A, B, and C is the same.
Application of Zeroth Law of Thermodynamics
The most common application of the zeroth law of thermodynamics is thermometers. The mercury expands as the temperature rises because the area of the tube stays constant. This change affects a height increase. Now, the variation in the mercury label’s height denotes temperature changes and essentially helps us measure it.
- The Zeroth law of thermodynamics is most frequently used in conjunction with the thermometer.
- To accurately measure temperature, a reference body and a temperature-dependent feature of that body are required. The change in that feature could be interpreted as a temperature change using zeroth law and examining certain characteristics that change in it to obtain an accurate temperature.
- This law gives definitions and guidelines for temperatures, which are potentially the most crucial system characteristics when tackling issues with thermal energy conversion.
- One can measure pressure with a constant gas volume thermometer, volume with a constant pressure thermometer, resistance with an electrical resistance thermometer, and length with a mercury in glass thermometer, for instance.
Examples of Zeroth Law of Thermodynamics
- The Zeroth Law of Thermodynamics is demonstrated by sweating in the human body. Sweat quickly evaporates in low atmospheric humidity. Our bodies provide the heat energy necessary for this sweat to evaporate, cooling the body as a result.
- The supply of hot or cold fluids for particular applications is common in many industries. The industrial thermometer measures the temperature of the fluid flowing through the pipe. To display the readings on the dial, the sensor that is attached to the thermometer must reach thermal equilibrium with the hot or cold fluids. This commercial thermometer is a good illustration of how the zeroth law of thermodynamics is used.
- Why does hot coffee turn cold if you don’t consume it right away?
This is caused by the zeroth law of thermodynamics. The room’s ambient air causes the hot coffee to lose heat.
Until the temperature of the coffee matches that of the room’s ambient air, it will continue to lose heat.
Let’s say that the air is 25 °C outside and that hot coffee is 70 °C inside. Coffee will then start to lose heat until it reaches a temperature of 25 °C. Coffee will lose heat until it reaches thermal equilibrium with the environment, in other words.
As a result, the example of the hot coffee cup illustrates the zeroth law of thermodynamics.
References
- R. Chang, “Physical Chemistry for the Chemical and Biological Sciences”, University Science Books, Sausalito, California (2000).
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/The_Four_Laws_of_Thermodynamics/First_Law_of_Thermodynamics/First_Law_of_ Thermodynamics
- Aston, J. and Fritz, J.J., Thermodynamics, and Statistical Mechanics, John Wiley and Sons, Inc., New York, 1959.
- Atkins, P.W. and Julio de Paulo, Atkins’ Physical Chemistry, Oxford University Press, UK, Indian Edition 9, 2011.
- https://byjus.com/jee/zeroth-law-of-thermodynamics/#:~:text=Similarly%2C%20another%20example%20of%20the,the%20temperature%20of%20the%20room.
- https://www.livescience.com/50833-zeroth-law-thermodynamics.html
