Wurtz reaction is very helpful in the fields of organic and organometallic chemistry for the formation of higher alkanes. Charles-Adolphe introduces the Wurtz reaction, in which a new carbon-carbon bond is created by coupling two alkyl halide molecules together in the presence of sodium metal in anhydrous ether or tetrahydrofuran. Symmetrical alkanes are prepared using this technique. Tetrahydrofuran is used as a solvent rather than ether in the case of alkyl and aryl fluorides, as well as aryl chlorides. In addition to sodium, other metals such as silver, indium, activated copper, zinc, and iron can be used in this reaction to produce alkanes.
The reaction in which aryl halides rather than alkyl halides are utilized then it is known as Wurtz Fittig reaction
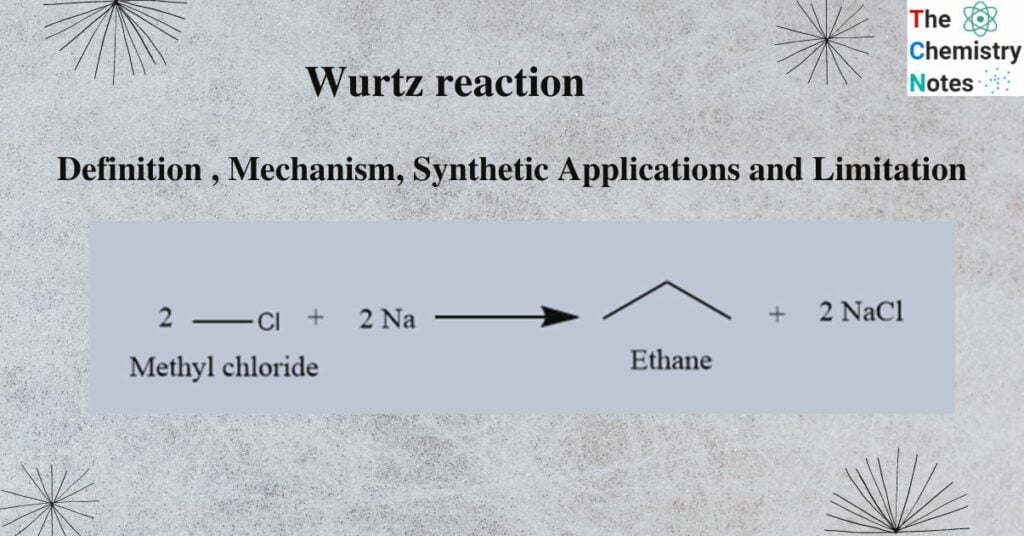
General reaction equation is as follows:
2 R-X + 2 Na → R – R + 2 NaX
Where, X = halogen (Cl, Br, I)
R = Alkyl group
Example: Two molecules of ethyl bromide reacts with sodium metal in presence of dry ether to give butane.
2 CH3CH2Br + 2 Na → C4H10 +2 NaBr
Reaction mechanism
The free radical species initiates the reaction mechanism. A free radical species represented by the symbol R• that is a component of a halogen-metal exchange is involved in this reaction mechanism.
The process involves the exchange of halogen and metal with the help of the radical species R, which results in the formation of a carbon-carbon bond as a result of a nucleophilic substitution reaction. The alkyl free radical created during the Wurtz reaction is strongly basic and can remove a proton from water, so it must be carried out under anhydrous conditions.
Step I: Formation of free radical due to transfer of a electron from sodium atom.
R-X + Na → R• + Na+X–
Step II: A carbonium ion is formed when a second sodium atom donates one more electron to the free radical.
R• + Na → R –Na+
Step III: Alkyl anion replaces the halide ion in the second alkyl halide molecule. The reaction proceeds via the SN2 reaction mechanism.
R−Na+ + R–X → R–R + Na+X–
Applications of Wurtz reaction
1. Higher alkanes with an even number of carbon atoms are prepared by using the Wurtz reaction.
Example: 2CH3Br + 2Na → C2H6 +2NaBr
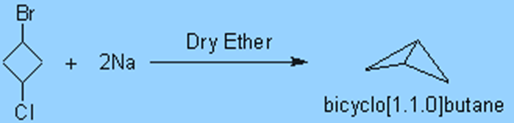
2. Through an intramolecular Wurtz reaction, constrained carbon skeletons such as bicyclobutane (bicyclo[1.1.0]butane) can be created.
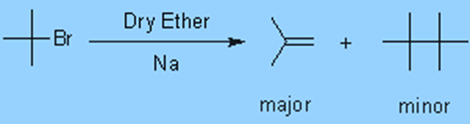
3. Isobutylene is the main end product of the Wurtz reaction when tert-butylhalides are involved. It’s because the SN2 mechanism is preferred to elimination. Due to steric hindrance, the SN2 step requires more activation energy.
Limitations of Wurtz reaction
- This reaction is not applicable to the preparation of lower alkanes like CH4. Wurtz reaction needs at least two carbon atoms to occur.
- Since sodium is involved, moisture is incompatible with this reaction. Furthermore, sodium is easily reacted with by oxygen and moisture, which can result in fire.
- With tertiary alkyl halides, the Wurtz coupling method frequently fails.
- Only symmetrical alkanes with an even number of carbon atoms can be created through this reaction.
- A mixture of alkanes is produced when different alky halides are used. Due to the fact that the mixture is typically difficult to separate, the Wurtz reaction is not a good way to create unsymmetrical alkanes.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
