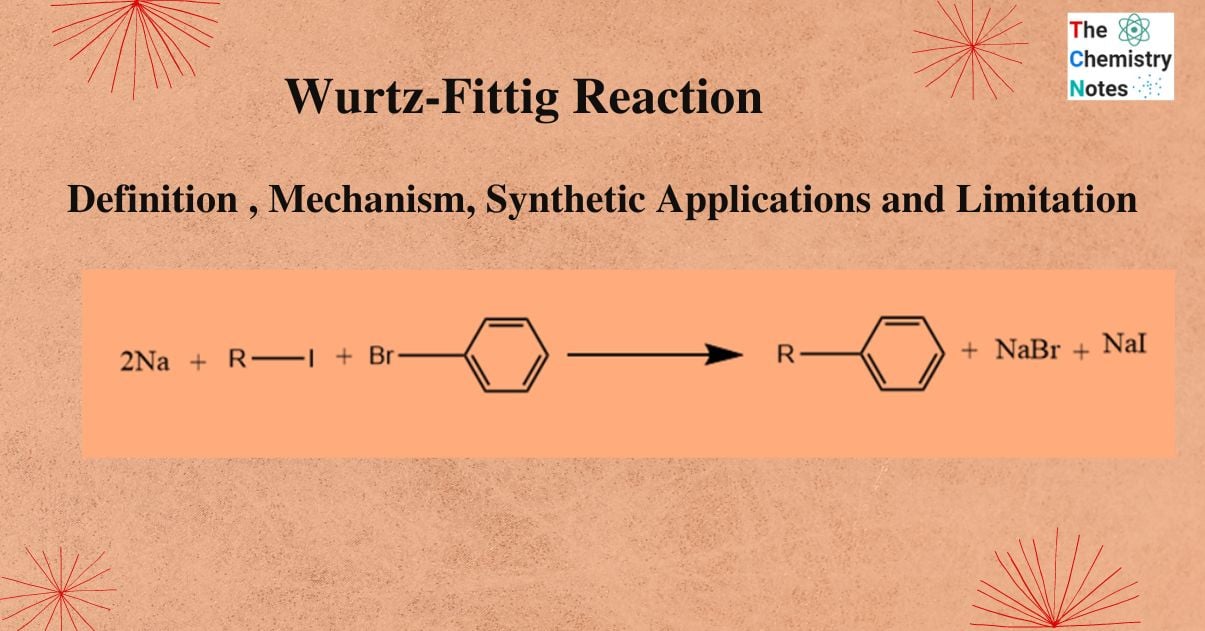
Wurtz-Fittig reaction is a chemical reaction that produces substituted aromatic compounds by coupling aryl halides with alkyl halides in presence of sodium metal, and dry ether.
The Wurtz reaction, in which two alkyl halides are coupled to form a new carbon-carbon bond, was first described by Charles Adolphe Wurtz in 1855. Later in the 1860s, Wilhelm Rudolph Fittig expanded the Wurtz method for coupling an aryl halide with an alkyl halide. So, this modification of the Wurtz reaction is named after both scientists and is considered a distinct process.
So, the Wurtz Fittig reaction produces alkylated aromatic hydrocarbons by coupling alkyl halide and aryl halide in the presence of sodium metal and dry ether. The overall reaction mechanism is as follows:
If the halide reactants are somehow distinct in their relative chemical reactivities, the reaction will function best for producing asymmetrical products. Producing the reactants from halogens from various periods is one way to accomplish this reaction. One way to do this is to use halogens from different periods to make the reactants. As aryl halides are typically made to be less reactive than alkyl halides, the likelihood that the alkyl halide will form the organosodium bond first and act as a nucleophile toward the aryl halide increases.
Wurtz-Fittig reaction mechanism
Wurtz-Fittig reaction involves two reaction mechanisms. They are as follows:
Radical mechanism
Alkyl and aryl radicals are formed using the radical approach, which is mediated by sodium. Then the substituted aromatic compound is formed by the combination of alkyl and aryl radical.
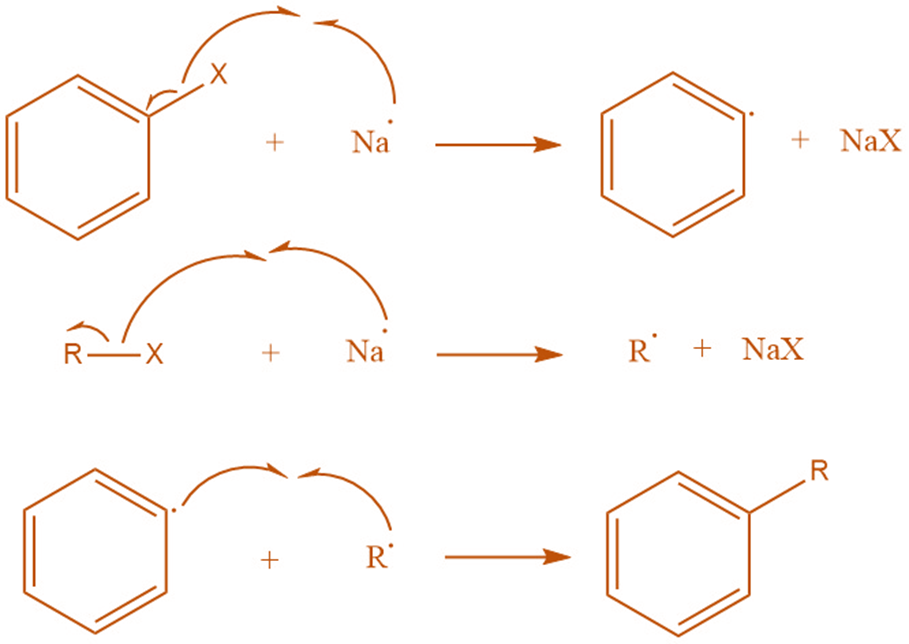
The observation of side products lends support to the free radical mechanism. For example, triphenylene is discovered by Bachmann and Clark to be one of the many byproducts of a reaction between sodium and chlorobenzene. They also demostrate that a free radical mechanism is the only way to account for the formation of triphenylene.
Organo-Alkali mechanism
In the organo-alkali approach, an aryl halide is reacted with sodium metal to produce an intermediate organo-alkali compound. This reaction mechanism involves the formation of an organo-alkali compound by the reaction of an aryl halide with sodium metal first, followed by the nucleophilic attack of alkyl halide.
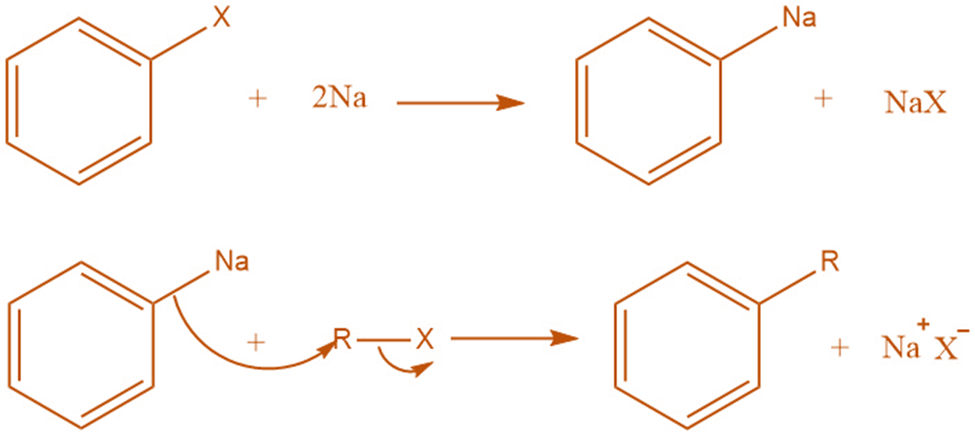
Indirect evidence demonstrating the formation of an organo-alkali intermediate during the reaction provides support to the organo-alkali mechanism. Shoruguin demonstrates the formation of 3-methylbutanoic acid by bubbling carbon dioxide through a solution of sodium and isobutyl bromide. This experiment also provides evidence for the organo-alkali mechanism.
Applications of Wurtz-Fittig reaction
1. This reaction is commonly used for the preparation of substituted aromatic compounds
2. It is also used for the synthesis of organo-silicon compounds in the laboratory.
3. For example, Calixarenes, t-ButylSilicon compounds, and vinylsilanes can be synthesized by using this reaction.
Limitation
- Due to the prevalence of side reactions like rearrangements and eliminations, the Wurtz-Fittig Reaction only has a limited range of applications.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
