Valence Shell Electron Pair Repulsion (VSEPR) Theory is a basic theory that predicts and explains the shape and bond angles of various molecules and ions with simple covalent bonds. This theory was proposed by Sedgwick and Powell initially in 1940 and developed by Gillespie and Nyholm in the year 1957 as VSEPR theory to predict the shape of the simple covalent molecules or ions containing more than two atoms.
This theory is based on the interactions of electron pairs in the valence shells of the atoms present in molecules. The electron pairs of the core atom in a covalent molecule interact with the least amount of repulsion. This results in molecules with minimal energy, maximal stability, and specific geometry.
Interesting Science Videos
Postulates of VSEPR Theory
This can be explained by the following postulates of the VSEPR theory:
- The valence pairs of electrons in the atoms connected to the center atom of a covalent molecule or ion have the least amount of repulsion, resulting in optimum stability and the least amount of energy.
- The number of valence pairs of electrons present in the central atom of the molecule determines the geometry of the molecule. The molecules or ions with 2,3,4,5,6,7 electron pairs in the central atom exhibit linear, trigonal planar, tetrahedral, trigonal bipyramidal octahedral, and pentagonal bipyramidal geometry.
- If the center atom has lone pair(s) in addition to bond pairs, the ideal shape of the molecule is distorted, resulting in a different bond angle than intended. The bond pair (b.p.) of electrons disperse and is shared by the two nuclei, whereas the lone pair (l.p.) of electrons are attracted to the single nucleus and occupies more space surrounding the central atom. As a result, the electron pairs’ repulsion around the center atom is in the following order: l.p.-l.p.>l.p.-b.p>b.p.-b.p.
- The extent of the repulsion between the electron pairs is determined by the difference in electronegativity between the central atom and the atom linked to it. The electronegativity of the atoms in the molecule also impacts the bond angles of the molecules, distorting the regular molecular geometry of iso-structural molecules (molecules with an equal number of bond pairs and lone pairs around the central atom).
- When the electronegativity of the central atom increases, electron bond pairs are attracted closer to it. This increases the repulsion between the bond pairs, increasing the bond angle.
- As the electronegativity of the atoms linked to the central atom increases, the electronegative atoms pull the bonded pairs of electrons closer together. This reduces the repulsion between the bond pairs, resulting in a decrease in the bond angle.
- The electron pairs responsible for molecular geometry are sigma (σ) electron pairs rather than pi (π) electron pairs, i.e., all the electron pairs of multiple bonds are treated as a single electron pair for electron pair interaction.
- The electron pairs responsible for the molecular geometry are sigma (σ) electron pairs but not pi (π) electron pairs i.e. all the electron pairs of multiple bonds are considered as a single electron pair for the interaction between the electron pairs.
Terms used in discussing the shape of the molecules
a. Bond Angle
The bond angle is the angle between a bonded atom, the central atom, and another bonded atom.
b. Lone Pair
The lone pair of an electron is a pair of valence electrons that are not shared with another atom and remains free.
c. Molecular Geometry
The three-dimensional arrangement of the bonded atoms in a polyatomic ion or molecule is considered as the molecular geometry.
d. Electron Pair Geometry:
The arrangement of the electron pairs around the central atom of a polyatomic ion or molecule is known as the electron pair geometry.
The primary difference between molecular geometry and electron pair geometry is that molecular geometry contains only bonded electrons but not unpaired electrons, while includes all bonded electrons and unpaired electrons.
Steps Involved in VSEPR Theory
The following are the steps to be considered to use VSEPR theory:
- The least electronegative atom should be chosen as the central atom or main atom (since it has the highest capacity to share electrons with the other atoms in the molecule).
- Draw a Lewis structure for the molecule or ion of choice.
- Count the number of electrons revolving around the central atom in the outermost shell.
- The total number of electrons belonging to other atoms and used in the bonds with the central atoms are also counted. Only the sigma (σ) electron pairs are responsible for the molecular geometry.
- The above two values are added to obtain the VSEPR number. Then, the molecular geometry of the molecules is determined based on the VSEP number.
(Note: To determine the molecular geometry, the lone pairs and the bonding pairs of electrons that are arranged around the central atom should be counted.)
What is the VSEP Number?
The VSEP number is a number that describes the shape of the molecules. This is described in the table given below:
| VSEP number | Shape of the molecule |
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | octahedral |
| 7 | pentagonal bipyramidal |
Molecular Geometry of Some Molecules based on the VSEPR theory
a. Regular Geometry
If the central atom in a molecule contains only bond pairs of electrons, a molecule will have a regular geometry. Gillespie and Nyholm calculated the regular geometries of the molecules containing 2 to 7 pairs of bonding electrons in the valence shell of a central atom.
Let us consider some of the examples of the molecules based on the electron pairs present in them:
I) Molecule containing two bonding electron pairs
e.g: BeF2 molecule
In the BeF2 molecule, Be is the central atom. The Lewis structure of the BeF2 molecule is given as:
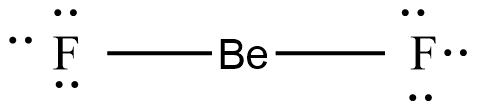
Be atom contains only two bonded pairs of electrons. The bound electrons are positioned at an angle of 1800, with the least amount of repulsion between them. As a result, the molecule is linear. Similarly, molecules with linear geometry include BeCl2, CO2, HgCl2, and others.
II) Molecule containing three bonding electron pairs
e.g: BCl3
The Central atom in this BCl3 molecule is B, which includes three bound electron pairs. The bond angle should be 1200 to have minimum repulsion between these three electron pairs. As a result, the molecule has a trigonal planar form.
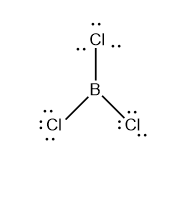
Similarly, the molecules such as CH2O, BF3, SO3, etc. also have three bonded electron pairs in each of their central atom and they also have trigonal planar geometry.
III) Molecule containing four bonding electron pairs
e.g: CH4 molecule
In methane, the central atom is carbon (C) which has four bonded electron pairs. It forms four equivalent bonds between four hydrogen atoms and these four bonds are directed towards the four corners of a regular tetrahedron. According to the VSEPR theory, a molecule with four equivalent bonds has the regular geometry of a tetrahedron with a bond angle of 109O.5′
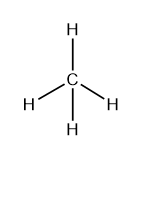
Similarly, the molecules such as CCl4, NH4+, etc. also have tetrahedral geometry.
IV) Molecule containing five bonding electron pairs
e.g: PCl5
The central atom in the PCl5 molecule is P, which has five bonding electron pairs. According to the VSEPR theory, all those bound electron pairs are directed toward the corners of a trigonal pyramid. Three of these five electron pairs are on the same plane, known as the equatorial plane, while the other two are in opposite directions. The bond angle between two equatorial electron pairs is 1200, while the bond angle between two axial electron pairs is 900.
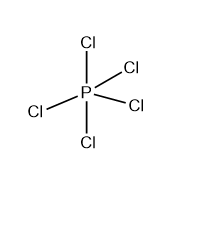
V) Molecule containing six bonding electron pairs
e.g: SF6
The central atom in this SF6 molecule is S which has six bonding electron pairs. These six bond pairs produce an equivalent bond with six fluorine atoms. According to the VSEPR theory, a molecule with six bound electron pairs must have a regular octahedral shape. The bond angle between two nearby electron pairs is 900, and the bond angle between two trans-electron pairs is 1800.
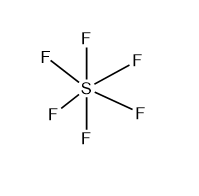
VI) Molecule containing seven bonding electron pairs
e.g: IF7
The central atom of this IF7 molecule is I, which has seven bonding electron pairs. According to the VSEPR theory, if a molecule’s center atom includes seven electron bond pairs, the molecule must have a regular shape of pentagonal bipyramidal. In this molecule, five electron pairs are in the same plane at an angle of 72⁰ while the other two electron pairs are perpendicular to the plane, and thus both electron pairs make an angle of 90⁰ with the plane.
Summarised table of the regular geometry of the molecules based on VSEPR theory
The summary of the above-discussed topic on the regular geometry of the molecules based on VSEPR theory is tabulated below:
| No. of electron pairs in the valence shell | The ideal shape of the molecule | Bond angle | Examples |
| 2 | Linear | 180⁰ | BeF2, BeCl2, CO2 |
| 3 | Trigonal planar | 120⁰ | BF3, BCl3, SO3, CH2O |
| 4 | Tetrahedral | 109.5⁰ | CH4, CCl4, NH4+ |
| 5 | Trigonal Bipyramidal | 90⁰, 120⁰ | PCl5 |
| 6 | Octahedral | 90⁰, 180⁰ | SF6 |
| 7 | Pentagonal bipyramidal | 72⁰, 90⁰ | IF7 |
b. Distorted geometry
The presence of one or more lone pairs of electrons in the central atom’s valence shell is the cause of the distorted geometry of the molecule. Here are some examples of such molecules:
I) NH3 molecule
Nitrogen is the central atom of ammonia (NH3), which contains five electrons in its valence shells. Three of these electrons are used to form bonds with three H atoms, yielding three bond pairs; the other two electrons are not involved in bonding and are referred to as lone pairs. According to the VSEPR theory, molecules with four electron pairs have tetrahedral structures. But in general, NH3 has a trigonal pyramidal structure due to the b.p. – b.p., and l.p. – b.p. repulsion, which causes the bond angle to decrease from 109.50 to 107.50.
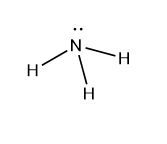
II) H2O molecule
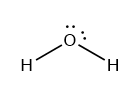
The central atom in a water molecule is oxygen (O), which includes four electron pairs. Two of these four pairs of electrons establish bonds with the hydrogen atoms and are known as bonding pairs, whereas the other two do not form bonds and are known as lone pairs. According to VSEPR theory, the geometry of the water molecule should be tetrahedral, but this geometry is deformed and V-shaped. This is due to the repulsions between l.p. – l.p., l.p. – b.p., and b.p. – b.p. Since there are l.p. – l.p.> l.p – b.p.> b.p – b.p. repulsions, the bond angle is reduced from 109.50 to 104.50.
III) CO2 and SO2 molecules
VSEPR theory only considers the sigma electron pairs. The CO2 molecule has two net electron pairs, one for each double bond, and should have a linear shape with a bond angle of 1800.
It has two bond pairs and one lone pair of electrons in S because the SO2 molecule contains one coordinate covalent bond and one covalent bond for a double bond. The shape of the SO2 molecule is bent with a bond angle of 117.50 due to the l.p.-b.p. and b.p.-b.p. repulsion as well as the l.p-b.p.>b.p.-b.p. repulsion.
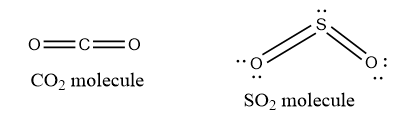
VSEPR Notation
The VSEPR notation provides a general formula for classifying chemical components based on the number of electron pairs surrounding the central atom. All species do not have the same molecular geometry. Consider carbon dioxide and sulfur dioxide, where one is linear while the other is bent.
Sometimes, the notation is expanded to include the lone pair of electrons. But generally,
- A is used to represent the central atom of the molecule.
- B or X is used to represent the number of atoms that are bonded to the central atom.
- E is used to represent the number of lone pairs of electrons on the central atom.
Importance of VSEPR Theory
I) Lewis structures are limited to two dimensions and only provide information about the number and types of bonds between the atoms. The VSEPR theory, on the other hand, is a three-dimensional model that describes the shape of molecules and the bond angle between atoms.
II) VSEPR theory is based on the idea that electrons will arrange themselves around the central atom to minimize repulsion that determines the specific geometry of the molecule.
III) It can predict the structure of nearly all compounds with the central atom, provided that it is not a metal.
Limitations of VSEPR Theory
Some limitations of VSEPR theory are as follows:
1. This theory fails to account for isoelectronic species or elements with the same number of species. These species may differ in shape despite having the same amount of electrons.
2. The VSEPR theory does not work for transition metal compounds. The transition metal elements in the d-block have relatively high atomic masses and tend to have stereochemically inactive electron pairs. As a result, VSEPR does not provide the right geometry for transitional metal complexes.
3. The VSEPR theory ignores the impact of orbital interactions on molecular structures.
4. Although VSEPR theory predicts that group-2 halides will have a linear structure, they have a bent shape. Quantum mechanics and atomic orbitals can give more sophisticated predictions when this VSEPR theory is inadequate.
References
1. Lee J. D. (1977). A new concise inorganic chemistry (3d ed.). Van Nostrand Reinhold. Retrieved May 12 2023 from https://archive.org/details/newconciseinorga00leej.
2. https://www.chem.uci.edu/~unicorn/old/H2A/handouts/PDFs/LectureB2.pdf
3. https://www.dalalinstitute.com/wp-content/uploads/Books/A-Textbook-of-Inorganic-Chemistry-Volume-1/ATOICV1-1-1-VSEPR-Theory.pdf.
4. Asmita’s Modern Approach to Chemistry-II by Daman Raj Gautam.
5. A New Comprehensive Chemistry (Grade XI) by Prof. Dr. P Wagley.
6. https://byjus.com/jee/vsepr-theory/
7. https://www.sciencedirect.com/science/article/pii/0898122186904384.
8. https://www.toppr.com/guides/chemistry/chemical-bonding-and-molecular-structure/vsepr-theory/.
