Vanadium is a chemical element that belongs to Group 5 (Vb) of the periodic table and is represented as ‘V’ in the periodic table. Found combined in various minerals, coal, and petroleum, vanadium is the 22nd most abundant element in Earth’s crust. It is the 23rd element of the periodic table. It is a transition metal that is found to the left of chromium, to the right of titanium, and to the upper right of niobium.
Vanadium is a rare, hard, ductile gray-white element found combined in certain minerals and used mainly to produce certain alloys. Vanadium resists corrosion due to a protective film of oxide on the surface. The elemental metal is uncommon in nature, but once separated, the creation of an oxide layer (passivation) helps to protect the free metal against further oxidation.
Interesting Science Videos
History of Vanadium
In the year 1801, Andrés Manuel del Rio, Spanish–Mexican scientist, naturalist and engineer discovered Vanadium for the very first time in the history.
Rio submitted vanadium ore samples and a letter outlining his procedures to the Institute de France in Paris, France, for testing and confirmation. Rio’s letter was destroyed in a shipwreck, and the Institute only got his samples, which included a brief note explaining how closely this new element, which Rio termed erythronium, resembled chromium.
Nils Gabriel Sefstrom, Swedish chemist, rediscovered vanadium in 1830, while analyzing the samples of iron from ore.
Sir Henry Enfield Roscoe isolated vanadium in 1867, during the reaction of vanadium trichloride with hydrogen gas.
Vanadium got it’s name after ‘Vanadis’ who is the Scandinavian Goddess of beauty, as vanadium form various multicolored beautiful compounds.
Occurrence of Vanadium
- Vanadium in native form is rare in nature.
- Vanadium compounds are found naturally in around 65 distinct minerals.
- It is the 22nd most abundant element in the earth crust.
- It may be found in crude oil, coal, oil shale (sedimentary rock), and tar sands. Vanadium is detected spectroscopically in the light of the sun and other stars.
- Vanadium in the form of their compounds exit in nature. It is mined mostly in South Africa, North West China and Eastern Russia.
- Burning fossil fuels emits an estimated 110,000 tons of vanadium into the atmosphere each year.

Isotopes of Vanadium
Vanadium has two isotopes; naturally occurring vanadium contains one stable isotope, 51V, and one radioactive isotope, 50V.
| Isotope | Natural abundance (atom %) |
|---|---|
| 50V | 0.250 (4) |
| 51V | 99.750 (4) |
- V-50, has a very low natural abundance (0.25%). V-50 is occasionally used for research purposes.
- V-51 is used in diabetes and metabolism studies.
Elemental Properties of Vanadium
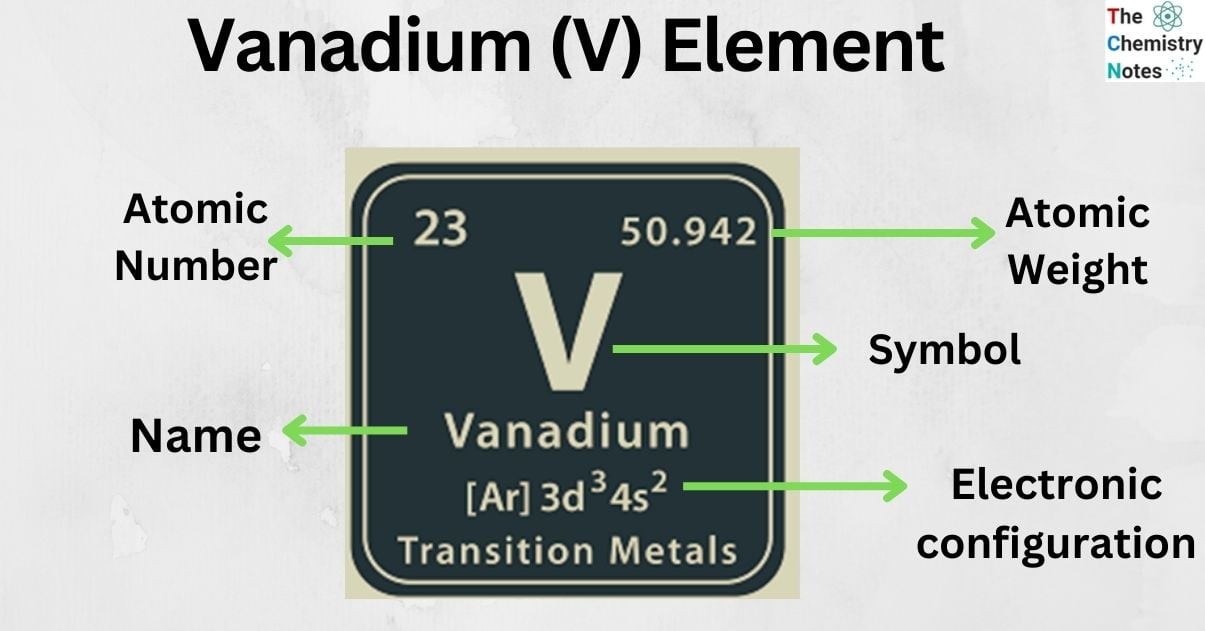
| Electronic Configuration | [Ar] 3d3 4s2 |
| Atomic Number | 23 |
| Atomic Weight | 50.9415 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 5, 4, d-block |
| Density | 6.1 grams per cubic centimeter at 20 °C |
| Ionic radius | 0.099 nm |
| Van der Waals radius | 0.197 nm |
| Electron shells | 2,8,11,2 |
| Electrons | 23 |
| Protons | 23 |
| Neutrons in most abundant isotope | 28 |
Physical Properties of Vanadium
- Vanadium is a hard, ductile metal with a silvery-gray appearance.
- It is electrically conductive and thermally insulating.
- It is tougher than most metals and has excellent corrosion resistance to alkalis and acids.
- Vanadium has a density of 6.11 grams per cubic centimeter. It has a melting point of 1910 ℃ and a boiling point of 3407 ℃.
- It is a medium hard and ductile metal.
- Vanadium is valuable in nuclear applications due to its high structural strength and low-fission neutron cross-section.
- Common oxidation states of vanadium include +2, +3, +4 and +5.
| Color/physical appearance | Silvery-white metallic |
| State at 20 ℃ | Solid |
| Melting point/freezing point | 1910°C, 3470°F, 2183 K |
| Boiling point | 3407°C, 6165°F, 3680 K |
| Density | 6.0 (g cm−3) |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.63 (Pauling Scale) 1.53 (Allen Scale) |
Chemical Properties of Vanadium
- Vanadium is moderately reactive.
- It does not react with oxygen in the air at room temperature, nor does it dissolve in water.
- Polymeric compounds known as isopolyvandates characterize aqueous chemistry. Under typical circumstances, V (IV) is most stable as halides and vanadyl ion (VO+2).
- It does not react with some acids, such as hydrochloric or cold sulfuric acid. But it does become more reactive with hot acids, such as hot sulfuric and nitric acids.
- Vanadium is special in that it acts like a metal in some cases, and as a non-metal in other cases.
Chemical Reaction of Vanadium
- Reaction of Vanadium with Oxygen
When heated, vanadium metal combines with excess oxygen, O2, to generate vanadium (V) oxide, V2O5. V2O5 is sometimes contaminated by other vanadium oxides when manufactured in this manner.
4V(s) + 5 O2(g) → 2 V2O5(s) [yellow-orange]
- Reaction of Vanadium with acids
In an acetic acid solution, sodium vanadate generates yellow decavanadate ions: [V10O28]6−, [V10O27(OH)]5−, [V10O22(OH)2]4−.
At pH values 1 > pH > 6 decavanadate ions are present.
VO43−(aq) + 25 H+(aq) ⇌ [V10O27(OH)]5−(aq) + 12 H2O(l)
At pH 1, V(V) exists as the light yellow dioxovanadium ions VO2+ in mineral acids.
VO43−(aq) + 4 H+(aq) ⇌ VO2+(aq) + 2 H2O(l)
- Reaction of Vanadium with Bases
Vanadium metal is resistant to molten alkali. In highly alkaline solutions (pH > 13), V(V) exists as colorless orthovanadate ions, VO43-.
- Reaction of Vanadium with Halogen
When vanadium is heated, it combines with fluorine, F2, to generate vanadium(V) fluoride.
2V(s) + 5F2(g) → 2VF5(l) [colourless]
- Reaction of Vanadium with Water
Vanadium does not react with water under normal conditions.
- Redox Reaction of Vanadium
Under acidic circumstances, V(V) is reduced to blue V(IV) (vanadyle ions) by reducing agents such as H2S and SO2.
VO2+(aq) + SO2(aq) → VO2+(aq) + SO42−(aq)
VO2+(aq) + 2 OH−(aq) → VO(OH)2(s) [brown]
Uses of Vanadium
- Vanadium is most often used as a steel addition, with around 80% of vanadium going into ferrovanadium, a steel additive. It is used to make rust-resistant, spring, and high-speed tool steels. It is also used to stabilize carbides in steels.
- Due to their strength, vanadium steel alloys are ideal for use as girders in construction as well as in the manufacture of tools, axles, and piston rods. Vanadium may be used to create steel alloys, as well as in space vehicles, nuclear reactors, and aircraft carriers, among other things.
- Vanadium is used to treat a variety of medical conditions, including diabetes, heart disease, and excessive cholesterol. It is also employed in the cathodes of batteries used in implanted cardioverter defibrillators.
- One use of ferrovanadium is improved corrosion resistance to alkaline reagents, sulfuric and hydrochloric acids.
- A little amount of vanadium metal (0.1 to 0.3 percent) interacts with the carbon in the steel to generate finely distributed carbides like V4C3, which boosts the mechanical strength of the alloy at high temperatures.
- In metallurgical operations, it is an excellent scavenger for removing the remaining vestiges of oxygen molecules.
- VO2 offers a wide range of applications in neuromorphic computing and space communications systems.
- Vanadium and its oxide compounds are frequently utilized as a chemical catalyst in organic and inorganic chemistry for the oxidation of naphthalene to phthalic acid, toluene to benzaldehyde, and aromatic hydrocarbon hydrogenation.
Health Effects of Vanadium
Vanadium is a trace element that is required by many animal species. Vanadium insufficiency is linked to growth retardation, poor reproduction, abnormal red blood cell formation and iron metabolism, and alterations in blood lipid levels. Health specialists are increasingly convinced that the metal can perform a similar role in humans.
- There is some evidence that large dosages of vanadyl sulfate (100 mg daily, yielding 31 mg elemental vanadium) may enhance how persons with type 2 diabetes use insulin, the sugar-processing hormone. According to the findings, high-dose vanadium may reduce blood sugar levels in persons with type 2 diabetes.
- Vanadium compounds have demonstrated intriguing biological effects, including anticancer activity, anti-amoebic activity, and therapeutic benefits in the treatment of several parasite disorders, when supplied at pharmaceutical levels. Additionally, it has been discovered that these substances have antiviral and antibacterial activities.
- Although vanadium compounds are not considered a major threat, employees exposed to vanadium peroxide dust experienced severe eye, nose, and throat discomfort.
- When vanadium levels are excessively high, it can have a range of negative impacts on human health. Vanadium absorption from the air can result in bronchitis and pneumonia.
Environmental Effects of Vanadium
The role of vanadium (V) in the functioning of land systems is quite diversified, since this element may have both positive and negative impacts on terrestrial species. However, high concentrations of vanadium in the environment pollute the soil and harm the majority of living species. A considerably higher amount of V disrupts plant physiological equilibrium, slowing biomass development and reducing yield dramatically. In turn, modest dosages of the proper vanadium ions can promote plant growth and development, exert cytoprotective actions, and efficiently improve the production of several physiologically useful chemicals.
- Vanadium pollution of soil is presently a major problem. Because to the complexities of V environmental chemistry, there is no easy method for limiting soil contamination and improving cleaning. Vanadium toxicity varies greatly depending on the chemical under examination.
- Vanadate ions have a detrimental impact on the phosphate-metabolizing system in most living organisms by inhibiting phosphatases, phosphodiesterases, ribonucleases, ATPases, and other enzymes. As a result, it is critical that vanadium(V) be reduced to a less soluble V(IV) form by inorganic processes such as H2S or by certain metal-reducing microbes. Vanadium may be reduced by the microbiota through detoxification or used as an electron acceptor during respiration.
- Vanadium may be found in algae, plants, insects, fish, and many other species. Vanadium bioaccumulates strongly in mussels and crabs, resulting in concentrations that are 105 to 106 times higher than those found in seawater.
Fun Facts about Vanadium
- Sefstrôm was granted the chance to name vanadium because he was largely recognized with its discovery. He picked the name Vanadis, which was connected with fertility and beauty in Old Norse mythology.
- During World War I, vanadium was employed to construct portable artillery pieces and body armor.
- Vanadate (a Vanadium compound) prevents steel from rusting. Vanadium foil is used to connect Titanium and steel since it is compatible with both metals.
- Vanadium has a variety of oxidation states that include purple, green, blue, and yellow.
- Vanadium can be used in the purification of uranium for nuclear usage.
Watch out the video about the interesting Chameleon Metal (Vanadium).
References
- B. Smith Hopkins, Chemistry of the Rarer Elements, 1923, D.C. Heath and Company, p205
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E11
- Dieter Rehder, Bioinorganic Vanadium Chemistry, 2008, Wiley
- J.W. Mellor, A Comprehensive Treatise on Inorganic and Theoretical Chemistry, Volume IX, 1929, Longmans, Green and Co., p714
- Sydney Marks, A Text-book of Inorganic Chemistry Volume VI. Part III., 1929, Charles Griffin & Company Limited, p12
- Yang J., Tengng Y., Wu J., Chen H., Wang G., Song L., Yue W., Zuo R., Zhai Y. Current status and associated human health risk of vanadium in soil in China. Chemosphere. 2017;171:635–643.
- https://www.webelements.com/vanadium/chemistry.html
- Vanadium: environmental hazard or environmental opportunity? A perspective on some key research needs: https://doi.org/10.1039/D0EM00470G
- Ulmer U., Asano K., Patryk A., Enoki H., Nakamura Y., Pohl A., Dittmeyer R., Fichtner M. Cost reduction possibilities of vanadium-based solutions—Microstructural, thermodynamic, cyclic and environmental effects of ferrovanadium substitution. J. Alloy. Compd. 2015;648:1024–1030. doi: 10.1016/j.jallcom.2015.07.110.
- https://www.lenntech.com/periodic/elements/v.htm#:~:text=Effects%20of%20vanadium%20on%20the%20environment&text=In%20mussels%20and%20crabs%20vanadium,which%20has%20several%20neurological%20effects.

