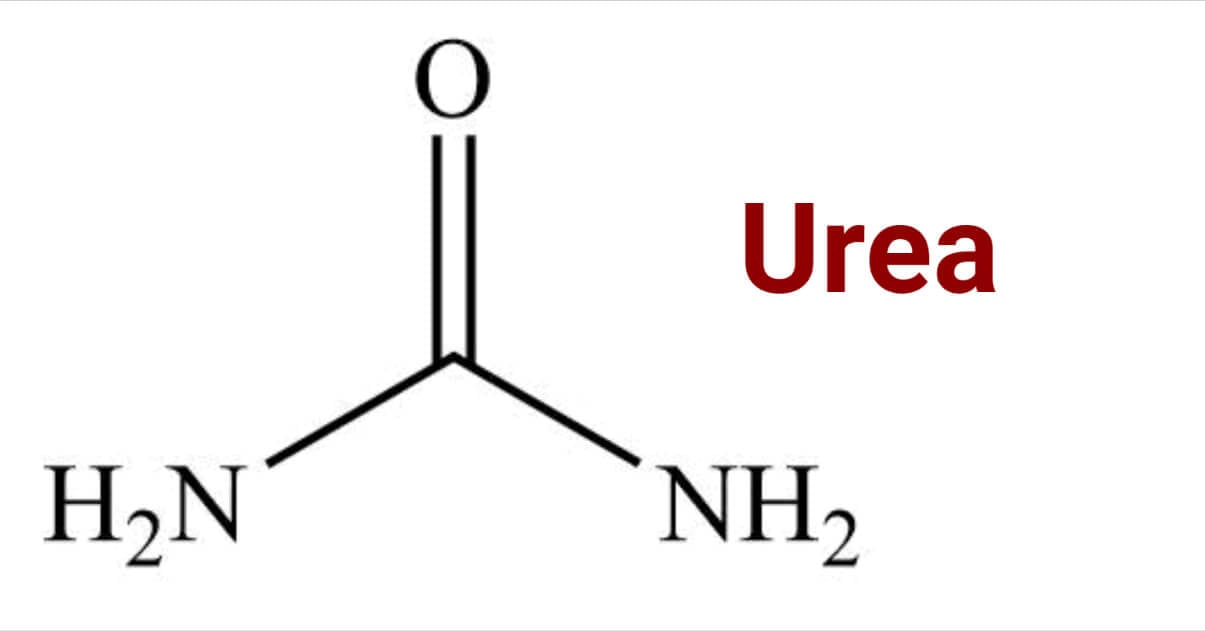
Urea is the nitrogenous byproduct of protein breakdown in mammals and some fishes. It is also called carbamide. It is not only the normal end product of the urine; it is also present in blood, bile, and milk and has a wide range of applications, including fertilizer and food supplements, as well as a raw material in the production of plastics and pharmaceuticals.
Replacement of both the -OH group of carbonic acid (H2CO3) with two amino groups (NH2) gives urea.
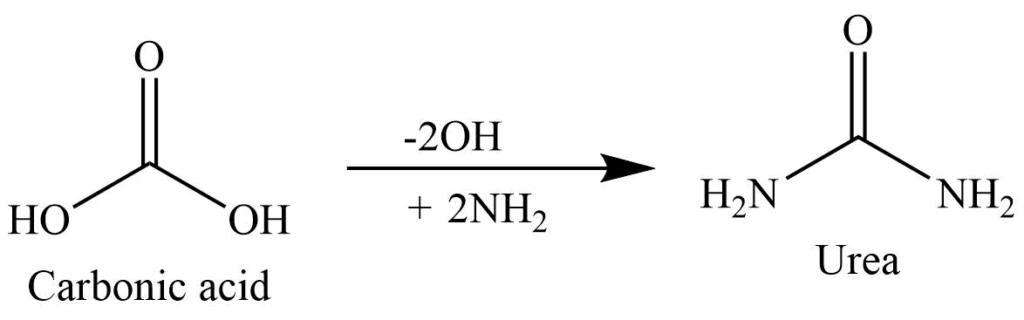
Preparation of Urea
From urine
Evaporation of the urine in small bulk, then treating it with nitric acid forms sparingly soluble urea nitrate. Urea is separated by the reaction of urea nitrate with barium carbonate. This is extracted with alcohol.
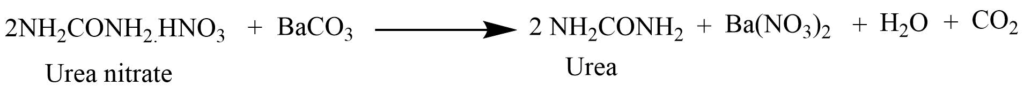
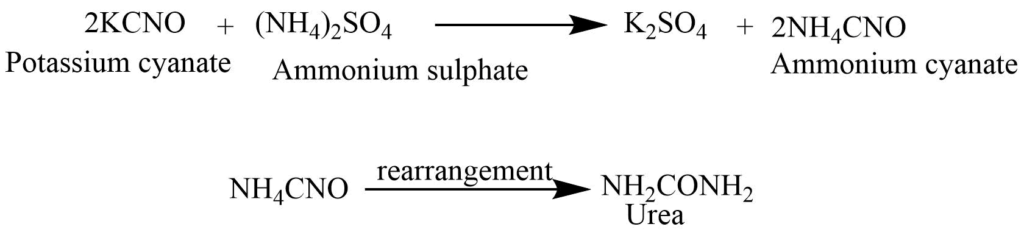
By heating the solution of potassium cyanate and ammonium sulphate to the dryness (Wohler synthesis)
The mixture of potassium cyanate and ammonium sulphate on heating to the dryness produces ammonium cyanate. Ammonium cyanate undergoes molecular rearrangement to form urea.
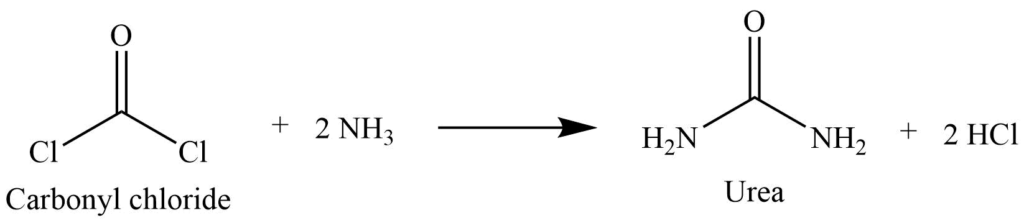
The action of ammonia on the carbonyl chloride (Laboratory preparation)
Carbonyl chloride reacts with two moles of ammonia to give urea.
By partial hydrolysis of cyanamide:
Cyanamide, obtained from the passing of nitrogen through heated calcium carbide at 800oC, on hydrolysis produces urea.
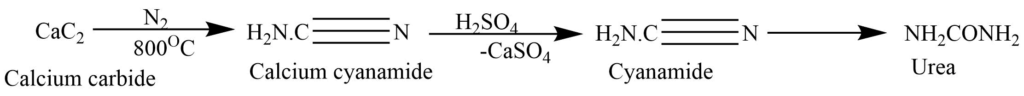
Physical properties of Urea
- Urea is a white crystalline solid.
- It is odorless and has a cooling taste.
- It melts at 132oC and decomposes before boiling.
- Urea is soluble in water but insoluble in ether.
- It acts as monobasic acid.
Chemical properties of Urea
It consists of the amide group attached to the amine. It can give the reaction of both groups. Mostly it behaves as an amide.
Basic nature
It acts as the monobasic acid and reacts with strong acid to form the salt. With the addition of nitric acid to the strong solution of urea produces white crystalline urea nitrate.
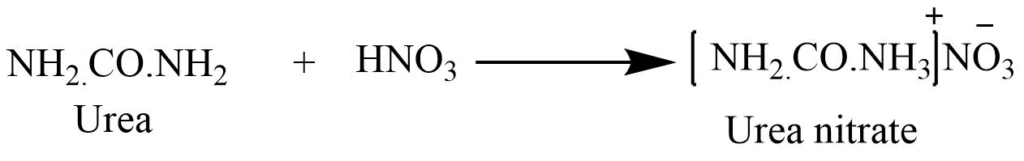
Hydrolysis
On boiling with dilute acid and alkali, it undergoes a hydrolysis reaction to give ammonia and carbon dioxide.
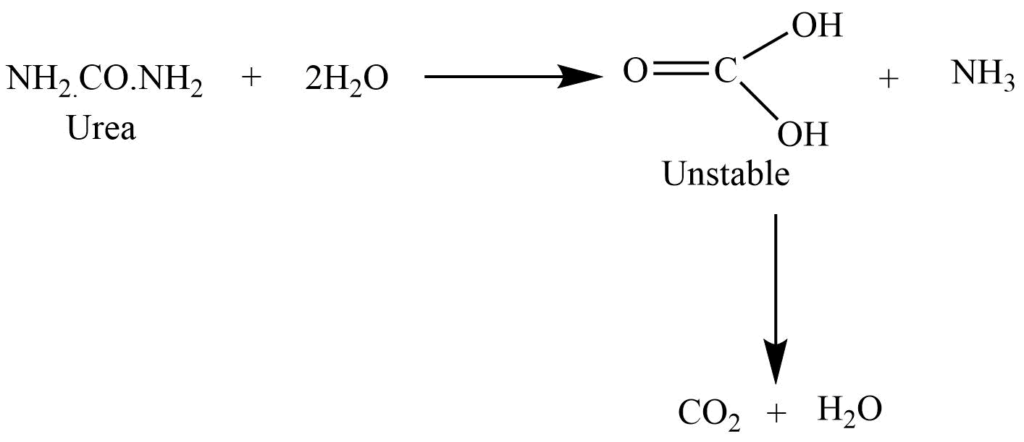
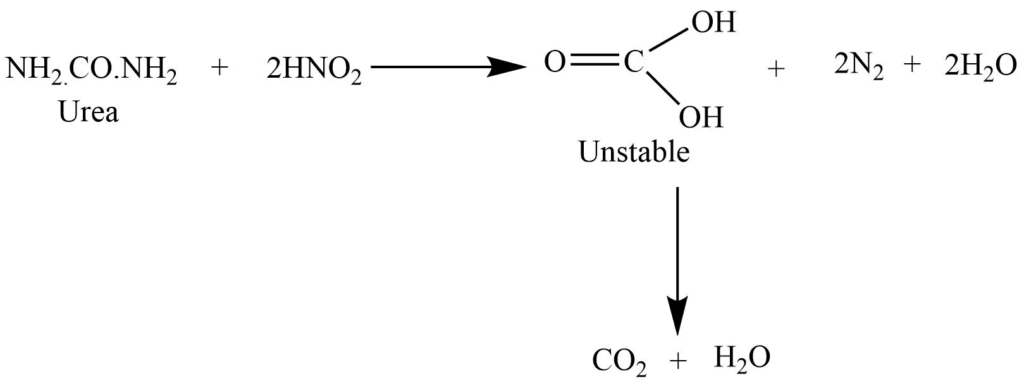
Reaction with the nitrous acid
The reaction with nitrous acid gives carbon dioxide, nitrogen, and water.
Acetylation reaction
It undergoes acetylation reaction on reaction with the acid chloride. In this process, acetylation occurs in one of the amide groups.
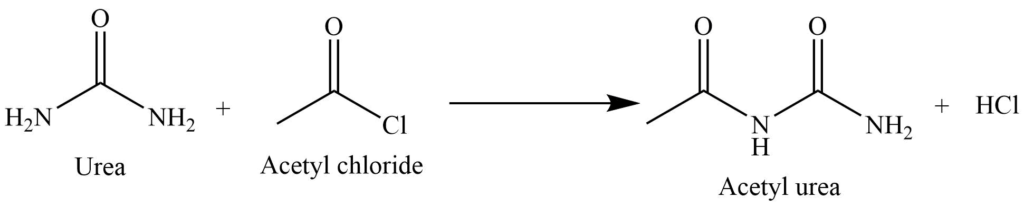
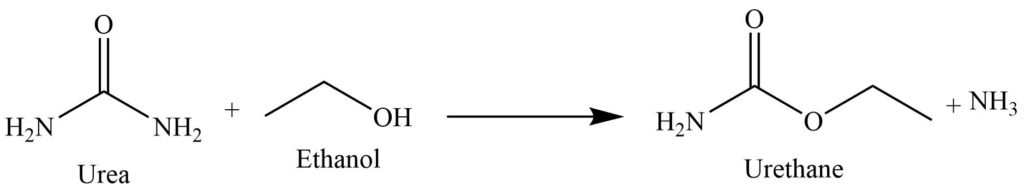
Reaction with ethanol
On heating with ethanol, it produces urethane.
Reaction with hydrazine
Urea reacts with hydrazine at 100oC to form the semicarbazide and ammonia.

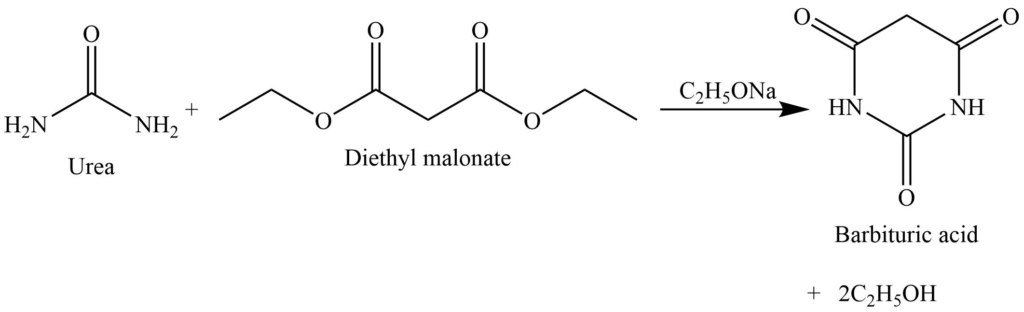
Formation of barbituric acid
The reaction of urea with the malonic ester (diethyl malonate) gives cyclic ureide i.e., barbituric acid. Which acts as a hypnotic.
Uses of Urea
- It is a type of fertilizer.
- Barbiturates can be obtained from urea. They act as hypnotics and sedatives.
- It is found in rehydrating ointment and hair removal creams.
- It acts as an explosive stabilizer.
- It is used in the production of dish soap.
- Urea is used as starting material for the production of plastic and drugs.
References
- https://www.britannica.com/science/urea
- https://www.toppr.com/ask/content/concept/urea-properties-and-uses-203034/
- https://byjus.com/chemistry/urea/
- https://thechemco.com/chemical/urea/
- https://byjus.com/urea-formula/
- https://www.vedantu.com/chemistry/urea
