UV spectroscopy involves the promotion of electrons (n, σ, π) from the ground state to a higher energy state. In U.V. spectroscopy, the excitation of the electron occurs in the range of 200-800 nm.
Interesting Science Videos
Principle of UV Spectroscopy
When the electromagnetic radiation of the correct frequency passes through the compound’s sample, some amount of energy is absorbed by the sample, which causes the excitation of an electron from the lower energy level to the higher energy level. The radiation on leaving the sample after absorption will be either less intense, or its intensity may be completely lost.
The absorption of light by a molecule is given by Beer-Lambert law as:
A= log Io/I = εc l
A= absorbance
Io = intensity of incident light
I= intensity of transmitted light
ε= molar excitation coefficient
c= concentration of solution in mole/cm-1
l= pathlength of sample
Types of Electronic Transitions
According to the molecular orbital theory, when a molecule is excited by energy absorption, the transition of electrons occurs from bonding to antibonding orbitals.
The various electronic transition is σ-σ*, n-σ*, π-π*, n-π*. The energy required for various transition obey the order of σ-σ*> n-σ*> π-π*> n-π*.
σ-σ* transition= It is a high-energy process. Saturated hydrocarbon like methane shows σ-σ* transition.
n-σ* transition = Saturated compounds containing one heteroatom show n-σ* transition. Here the transition from nonbonding orbital to sigma antibonding orbital takes place. This type of transition is sensitive to hydrogen bonding. Hydrogen bonding shifts absorption towards a lower wavelength.
CH3Cl= 172-175 mμ.
CH3I= 258 mμ. ( this is due to the low electronegativity of iodine)
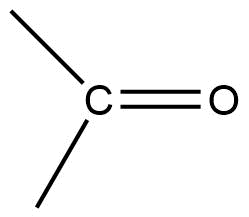
π-π* transition= Molecules having double and triple bonds show π-π* transition. This type of electronic transition normally occurs at lower energy.
e.g. Ethylene = 175 nm
n-π* transition = In this transition, one electron of the heteroatom gets excited to π antibonding orbital. This process requires low energy.
π-π*= 180 nm
Selection rules
The various electronic transitions are governed by different restrictions called selection rules. They are:
- Transitions involving a change in the spin quantum number of an electron during the transition, do not occur.
- Transition between the orbital of different symmetry does not occur. e.g., n-π* transition is symmetry forbidden.
The intensity of absorption is directly proportional to the transitional probability. An allowed transition will have an εmax value greater than 1000.
Different terms used in UV Spectroscopy
- Chromophores: Chromophores are the compounds that are responsible for imparting color to the compounds. They absorb the light in the U.V. region. E.g. nitro group present in the nitro compounds are the chromophore which imparts a yellow color to these compounds.
- Auxochromes: Auxochromes are colour-enhancing group. This group doen’t itself act as the chromophore but their presence shifts the absorption band towards the longer wavelength. e.g., -OH, -OR, -NH2 etc.
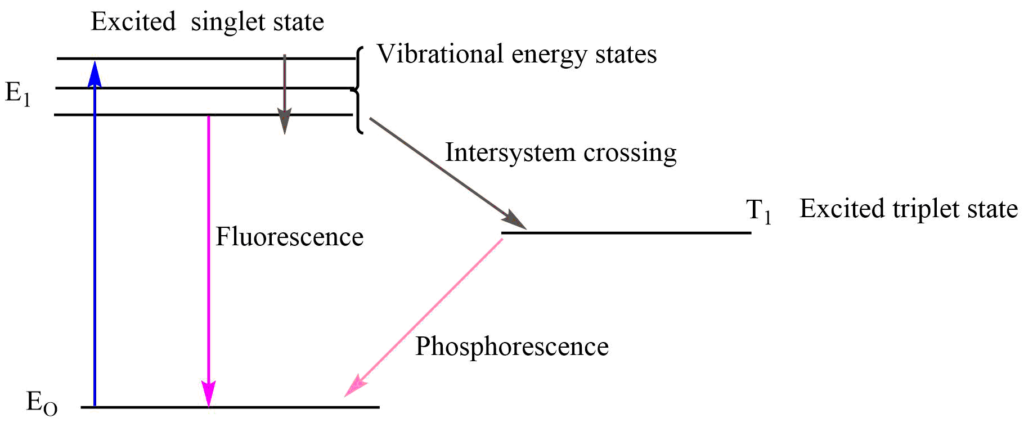
- Fluorescence: Fluorescence is the process by which a molecule emits light of a longer wavelength after absorbing different light of a short wavelength. The fluorescence process stops when the irradiating light is removed.
- Phosphorescence: The phosphorescence involves the continuous emission of radiation of longer wavelength when the irradiating light is removed.
Absorption and Intensity Shifts
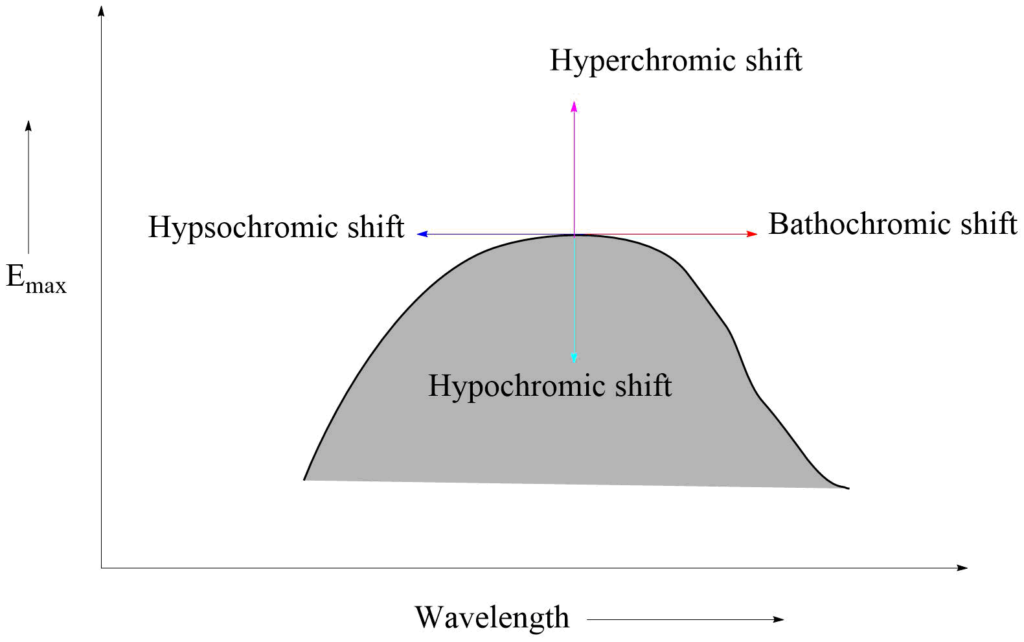
Bathochromic effect: It is an effect by which the absorption maximum is shifted towards a longer wavelength due to the presence of an autochrome or by a change of solvent. Bathochromic shifts are also called red shifts.
Hypsochromic shifts: This is the effect by which the absorption maximum is shifted towards a shorter wavelength. It is also called blue shift. It may be caused by the removal of conjugation and changing the solvent’s polarity.
Hyperchromic effect: It is an effect due to which the intensity of absorption maximum increases Emax increases. B-band for pyridine at 275 mμ, Emax 2750 is shifted to 262mμ, Emax 3560 for 2-methyl pyridine. The introduction of autochrome usually increases the intensity of absorption.
Hypochromic effect: It is defined as an effect due to which the intensity of absorption maximum decreases, i.e., extinction coefficient Emax decreases. The introduction of the group which distorts the geometry of the molecule causes the hypochromic effect.
Types of absorption bands
- K- bands= K- bands originate due to π-π* transition from a compound containing a conjugated system. Such types of bands arise in compounds like dienes, polyenes, and enones.
- R- bands= Such types of bands originate due to the n-π* transition of a single chromophoric group and have at least one lone pair of electrons on the hetero atom. R-bands are also called forbidden bands.
- B-bands= This type of band arises due to π-π* transition in aromatic or hetero-aromatic molecules.
- E-bands= Such bonds originate due to the electronic transitions in the benzenoid system of three ethylenic bonds, which are in closed cyclic conjugation.
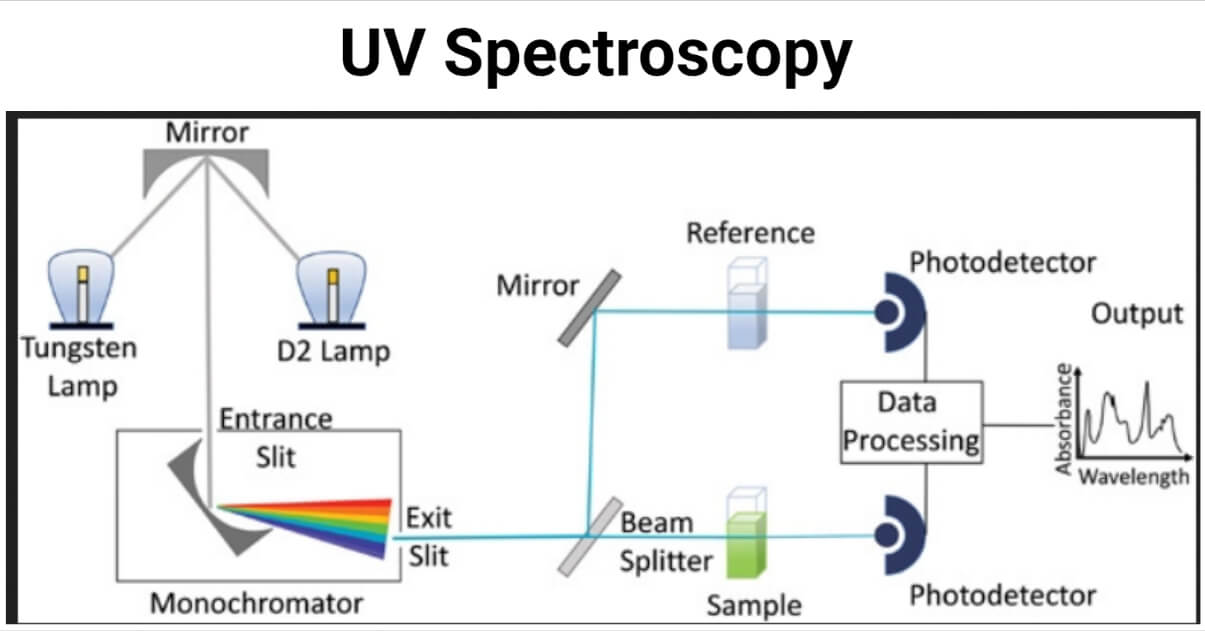
Instrumentation of UV Spectroscopy
UV Visible spectroscopy is a type of absorption spectroscopy in which the molecule absorbs light in the U.V-Visible range and excites its electrons from the ground state to a higher energy state.
Two types of U.V-Visible spectroscopy
1. Single beam spectroscopy
In a single beam spectroscopy monochromator, the sample and detector are arranged in series in the single beam configuration. Here the monochromator light with intensity Io is passed through the sample causing the excitation of electrons from lower energy to a higher energy state.
2. Double beam spectroscopy
In double beam spectroscopy, the splitter or chopper splits the monochromatic light into two beams one passes through the sample while the other passes through reference.
The main parts of the U.V- Visible spectroscopy include:
1. Source of U.V light
2. Monochromator
3. Chopper
4. Sample container
5. Detectors
5. Amplifier
6. Recorder
Source of U.V. light
H2-D2 lamp
H2-D2 lamp is used as the source of radiation which emits light within the range of 300-375 nm. In these lamps, quartz windows are typically used because the glass absorbs light below 375 nm.
Tungsten filaments
Tungsten filament is used as the source of visible light. This type of lamp is used in the wavelength range of 375 – 800 nm and covers a wide range of wavelengths. Tungsten-halogen lamps used in modern spectrometers have high lifetime than that of normal tungsten lamps. They are very efficient and cover the wavelength range up to the ultraviolet region.
Monochromator
It converts the polychromatic light into monochromatic light. It consists of different parts they are as follows:
- Entrance slit
- Collimating lens
- Grating or prism
- Reflecting lenses
- Exist lens
Polychromatic light enters the monochromator through the entrance slit. The beam is collimated by a collimating lens and dispersed by prism or grating. The reflecting lens reflects the beam with a particular wavelength towards the exit slit. By adjusting the position of the grating or exit slit, radiation with a particular wavelength leaves the monochromator through the exit slit.
Chopper
It splits monochromator light into two beams, one passes through the sample while the other passes through reference.
Sample container
The sample and reference solution containers must be radiation-transparent so that the radiation will pass through them. For UV-Visible spectroscopy, quartz or fused silica cuvettes are used as a sample container.
Detectors
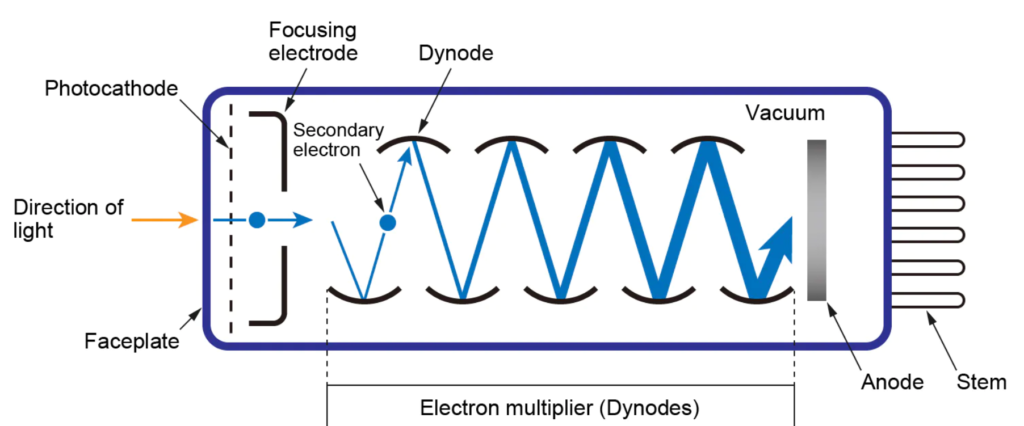
Photomultipliers tubes
Photomultiplier tubes are commonly used detectors in UV-visible spectroscopy and are sensitive to UV-Visible radiation. They have a fast response time and consist of a photoemissive cathode, several dynodes, and the anode. It is limited to measuring low-power radiation.
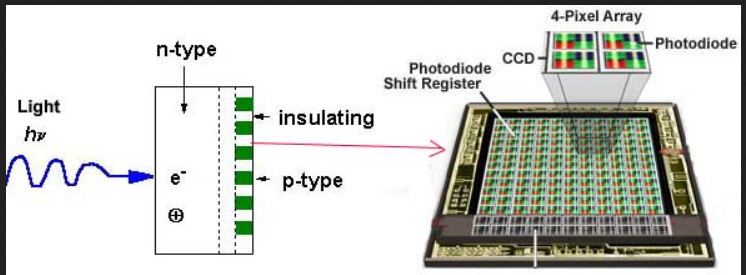
Photodiode arrays
They are the multichannel photon detector capable of measuring all elements of a beam of dispersed radiation simultaneously. They are beneficial for recording UV-Visible absorption spectra of samples passing through a sample flow cell quickly, such as in an HPLC detector.
Charged-coupled detectors
They are silicon-based multichannel array detectors and are similar to a diode array detector. Instead of the diode, they consist of an array of photo capacitors and are extremely sensitive to light.
Amplifier
Amplifiers amplify the signal from the detector many times to produce a clear and readable signal.
Recorder
It records all data and produces the spectrum of the desired compound.
Applications of UV Spectroscopy
- Detection of functional groups: U.V. spectroscopy is used to detect the presence and absence of chromophores. The absence of a band at a particular wavelength indicates the absence of a particular group in this compound.
- Extent of conjugation: The extent of conjugation in polyenes can be estimated by using U.V spectroscopy methods.
Conjugated and nonconjugated compounds can be distinguished by using U.V methods.
- Identification of unknown compounds: Unknown compounds can be identified by comparing their spectra with the spectra of known compounds. If two spectra coincide, these compounds must be identical otherwise different.
- Determination of configuration of geometrical isomers: Cis-alkenes absorb at different wavelengths as compared to their corresponding trans isomers. Cis and Trans isomers can be distinguished by using U.V spectroscopy.e.g.,
Cis-stillbene λmax = 283 mμ.
Trans-stillbene λmax= 295.5 mμ.
- Identification of compounds in different solvents: Sometimes, the structure of the compound changes with a change in a solvent which can be determined by using U.V spectroscopy.
Chloral hydrate shows an absorption maximum at 290mμ in hexane, while in aqueous solution, the absorption disappears. This is due to the presence of the carbonyl group in hexane solution as its structure is present as CCl3.CHO.H2O, whereas in an aqueous solution, it is present as CCl3.CH(OH)2.
- It is also used in the pharmaceutical analysis process and bacterial culturing.
References
- Silverstein, R. M., Webster, F. X., & Kiemle, D. J. (2005). Spectrometric identification of organic compounds. Hoboken, NJ: John Wiley & Sons.
- https://byjus.com/chemistry/uv-vis-spectroscopy/
- https://byjus.com/chemistry/principle-of-uv-visible-spectroscopy/
- https://www.indiastudychannel.com/resources/146681-Principle-working-and-applications-of-UV-spectroscopy.aspx
- https://www.pharmatutor.org/pharma-analysis/analytical-aspects-of-uv-visible-spectroscopy/applications.html
- https://microbenotes.com/uv-spectroscopy-principle-instrumentation-applications/
- https://www.technologynetworks.com/analysis/articles/uv-vis-spectroscopy-principle-strengths-and-limitations-and-applications-349865
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/10%3A_Spectroscopic_Methods/10.03%3A_UV_Vis_and_IR_Spectroscopy
- https://jascoinc.com/learning-center/theory/spectroscopy/uv-vis-spectroscopy/instrumentation/
- https://teaching.shu.ac.uk/hwb/chemistry/tutorials/molspec/uvvisab3.htm
- https://www.deshbandhucollege.ac.in/pdf/resources/1585214485_PHY(H)-VI-NANO_MATERIAL-13-AJAYPRATAP.pdf
- https://onlinelibrary.wiley.com/doi/10.1002/cjce.23344
- https://www.engineersgarage.com/ccd-charge-coupled-devices/
- https://www.globalsino.com/EM/page4
- https://www.matsusada.com/application/ps/photomultiplier_tubes/

helpful for my end-term university exam.