Transition metal complexes, also known as coordination complexes, are molecules with groups organized around a central metal ion. In some ways, these are similar to “lego-molecules,” as they can be easily constructed from smaller pieces and sometimes converted into new molecules by swapping out old parts for new ones. One of the reasons these chemicals are so effective in industrial and biological catalysis is their quick construction and disassembly.
Normally, transition metals form coordinate covalent bonds, a type of Lewis acid-base interaction in which a donor (Lewis base) contributes both electrons in the bond to an electron acceptor (Lewis acid). The Lewis acid in coordination complexes is frequently a transition metal or inner transition metal, while the main group elements can also form coordination compounds.
Interesting Science Videos
Complex Ion Formation in Transition Metals
All transition metals have the property of forming complexes.
A complex is a transition metal atom or ion surrounded by ligand molecules or ions. Ligands donate a pair of electrons to create co-ordinate bonds with transition metals.
A covalent bond is one in which both electrons come from the ligand. The Lewis base donors, known as ligands, can be any chemical—atoms, molecules, or ions. They must only have one or more electron pairs that can be donated to the center metal. Most commonly, this involves a donor atom with a single electron capable of forming a coordination bond with the metal.
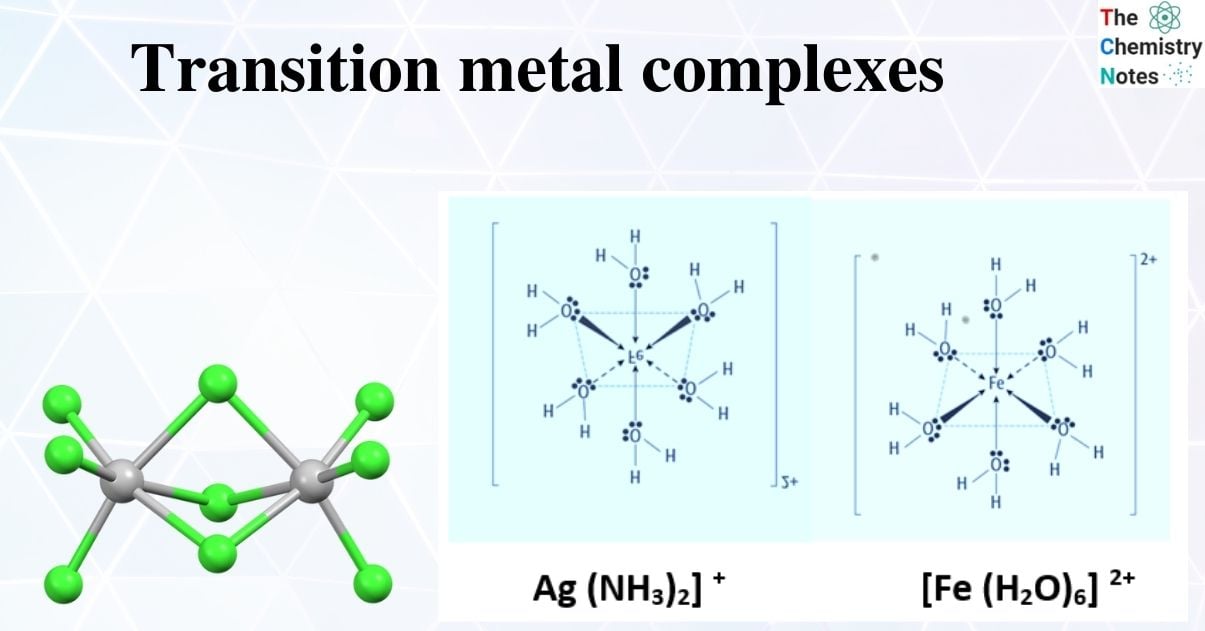
The coordination sphere is made up of the central metal ion or atom and its ligands. The coordination sphere is enclosed by brackets in a formula; species outside the brackets are not included in the coordination sphere. The number of donor atoms linked to the central metal ion or atom determines its coordination number.
The silver ion in [Ag (NH3)2] + has a coordination number of two. The coordination number for the copper (II) ion in [CuCl4]2 is four, while the coordination number for the cobalt (II) ion in [Co (H2O)6]2+ is six. Each of these ligands is monodentate, which comes from the Greek for “one-toothed,” and connects to the center metal via only one atom. The number of ligands and the coordination number are both equal in this scenario.
Complex Ion Naming
The complexes’ nomenclature is based on a scheme proposed by Alfred Werner, a Swiss chemist and Nobel winner whose pioneering research more than a century ago provided the groundwork for a better understanding of these chemicals. A complex ion’s name must include:
The number of ligands – A complex ion’s name begins with the number of ligands that make covalent bonds with the core atom.
The ligand type – The name of the ligand will appear after its number is supplied. If there are two or more types of ligands, they will be named alphabetically.
The central atom – At the end, the name of the central transition metal atom remains unchanged. Remember to include the oxidation number.
Rules for Naming Complexes
- If the coordination complex is ionic, name the cation first and the anion second, using standard nomenclature.
- First, identify the ligands, then the central metal. The ligands should be named alphabetically. Negative ligands (anions) are named by appending -o to the group’s stem name. The name of the molecule is used for the majority of neutral ligands. Aqua (H2O), amine (NH3), carbonyl (CO), and nitrosyl are the four most common exceptions (NO). For example, [Pt (NH3)2Cl4] is diamine-tetrachloro platinum (IV).
- If there is more than one ligand of a given type, the number for the prefixes di– (for two), tri– (for three), tetra– (for four), penta– (for five), and hexa– (for six). When the name of the ligand already includes di-, tri-, or tetra-, or when the ligand name begins with a vowel, the prefixes bis- (for two), tris- (for three), and tetrakis- (for four).
- When the complex is a cation or a neutral molecule, the central metal atom’s name is spelled precisely like the element’s name and is followed by a Roman numeral in parenthesis to denote its oxidation state. When the complex is an anion, the suffix -ate is appended to the metal’s stem, followed by the Roman number designation of its oxidation state.
- In cases where the English name of the metal is inappropriate use latin names. For example, ferrate replaces ironate, plumbate replaces leadate, and stannate replaces tinate. The metal’s oxidation state is govern by the charges of each ligand and the total charge of the coordination complex
Charges on Complexes
The charge on a complex is determined by the transition metal’s oxidation state and the charge on the ligand.
Here are some examples of calculation of complex ion’s charge:
[Co(NH3)]
The total charge is “3+” because the cobalt has an oxidation state of +3 and the ammonia molecules are neutral.
[Cu(Cl)4]
Because the copper has an oxidation state of +2, and each of the four chloride ions has a charge of “-1,” the total charge is “2-.”
Transition Metal Complexes with water & ammonia molecules
- Neutral ligands examples include water and ammonia molecules.
- Both ligands contain a single electron capable of forming a dative covalent connection with the central metal ion.
- This is the lone pair on the oxygen atom in water.
- It is the lone pair on the nitrogen atom in ammonia.
- Because water and ammonia are tiny ligands, six of them can fit around a central metal ion, each giving a single pair of electrons, resulting in six dative bonds.
- A complex’s coordination number is the number of dative bonds in between the central metal ion and the ligands.
- Because there are six dative bonds, the complex’s coordination number is six.
- Complexes with the coordination number 6 are octahedral in form.
- A complex’s overall charge is the sum of the charges on the central metal ion and the charges on each of the ligands.
- A compound with a core metal ion of cobalt (II) or copper (II) and water or ammonia molecules as ligands will have an overall charge of 2+.
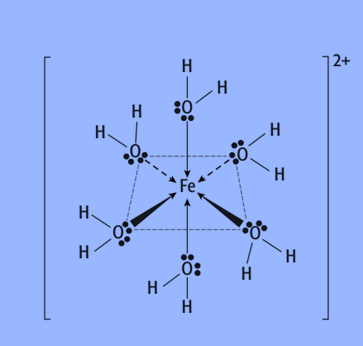
![[Fe (H2O)6] 2+](https://scienceinfo.com/wp-content/uploads/2022/10/image-37.png)
[Fe (H2O)6] 2+; the complex ion formed between a Fe2+ ion and six water molecules
Transition Metal Complexes with hydroxide & chloride ions
- Negatively charged ligands include hydroxide and chloride ions.
- Both ligands contain a single electron capable of forming a dative covalent connection with the central metal ion.
- Because hydroxide ligands are tiny, six of them can fit around a core metal ion, resulting in a complex with a coordination number of six.
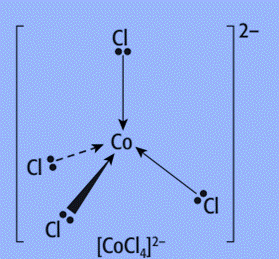
- Because chloride ligands are big, only four of them will fit around a central metal ion.
- Complexes containing four chloride ligands will have a coordination number of four. Complexes containing four chloride ligands generate tetrahedral complexes.
- Hydroxide ligands, on the other hand, will produce octahedral complexes.
- A compound containing cobalt (II) or copper (II) as the central metal ion and chloride ions as the ligands will have an overall charge of 2-.
- The charge of the center metal ion is 2+.
- Each chloride ligand is positively charged.
- Because the combination contains four chloride ligands, the overall negative charge is 4.
- The net positive charge is 2+.
- As a result, the complex’s total charge is 2.
- A compound with a core metal ion of cobalt (II) or copper (II) and hydroxide ions as ligands will have no overall charge.
- The charge of the center metal ion is 2+.
- Each hydroxide ligand is positively charged.
- Because the combination contains two hydroxide ligands, the overall negative charge is 2.
Structure of Transition Metal Complexes
| Coordination Number | Molecular Geometry | Example |
| 2 | linear | [Ag (NH3)2] + |
| 3 | trigonal planar | [Cu (CN)3] 2− |
| 4 | Tetrahedral (d0 or d10), low oxidation states for M | [Ni (CO)4] |
| 4 | square planar (d8) | [NiCl4]2− |
| 5 | trigonal bipyramidal | [CoCl5]2− |
| 5 | square pyramidal | [VO (CN)4]2− |
| 6 | octahedral | [CoCl6]3− |
| 7 | pentagonal bipyramid | [ZrF7]3− |
| 8 | square antiprism | [ReF8]2− |
| 8 | dodecahedron | [Mo (CN)8]4− |
| 9 and above | more complicated structures | [ReH9]2− |
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- Cotton, F. Albert; Wilkinson, Geoffrey (1988). Advanced Inorganic Chemistry ( 5th ed.). New York: Wiley-Interscience. ISBN 0-471-84997-9.
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/6-inorganic-chemistry-a-level-only/6-2-properties-of-transition-elements-a- level-only/6-2-3-transition-metal-complexes/
- https://courses.lumenlearning.com/chemistryformajors/chapter/ coordination-chemistry-of-transition-metals- 2/
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_(OpenSTAX)/19%3A_Transition_Metals_and_Coordination_Chemistry/19.2%3A_Coordination_Chemistry_of_Transition_Metals
