Titration is a typical quantitative/chemical analysis method used in laboratories to determine the unknown concentration of a known reactant (analyte). The method is based on a chemical reaction between a standard solution (titrant) and an analyte solution.
Titration is one of the earliest ways of determining content in chemistry. Titration is often used in chemical analysis. On the one hand, a titration is relatively simple and quick to do; on the other hand, the titration produces a very accurate measurement result after only a few minutes – under ideal conditions.
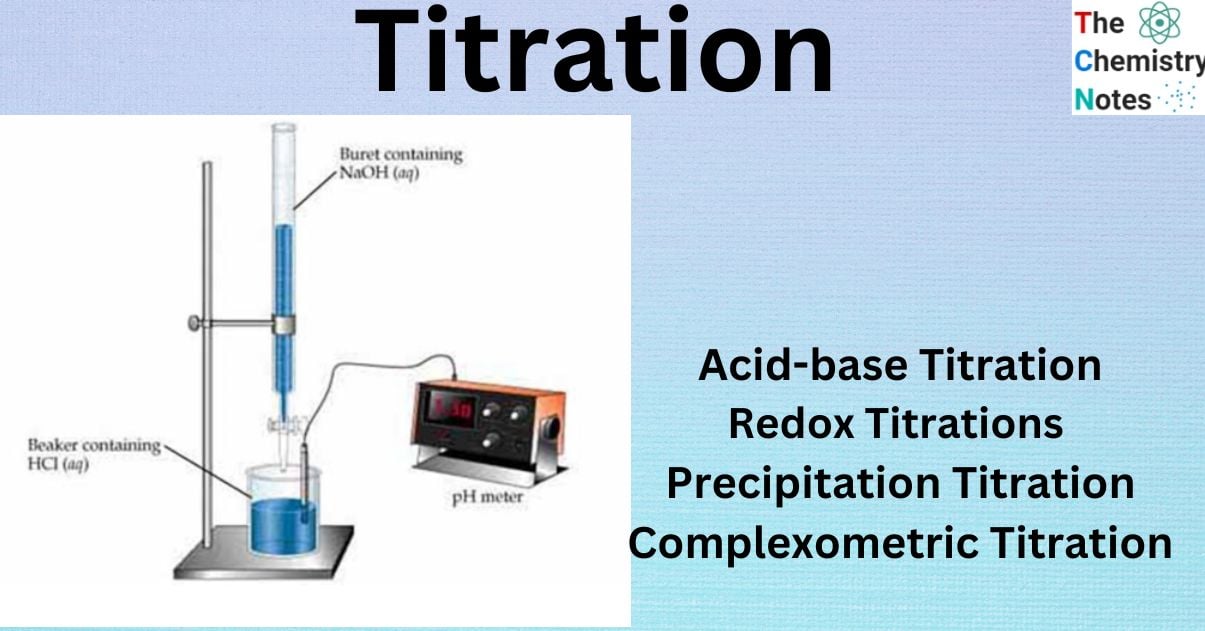
What is Titration?
Titration, commonly known as titrimetry, is a chemical qualitative analytical technique for determining the concentration of an analyte in a mixture. Titration, commonly known as volumetric analysis, is an important technique in the field of analytical chemistry.
- Titration is a method of determining the concentration of a solution by reacting a known volume of that solution with a known concentration of another solution.
- To determine the concentration of an acid solution, titrate the acid solution with a known concentration of a base solution.
- Titrate a base of unknown concentration with an acid of known concentration.
Procedure of Titration
- In a beaker, a measured volume of an acidic or basic solution of unknown concentration is placed. The electrodes of a pH meter are immersed in this solution, and the solution’s initial pH is read and recorded.
- A burette is filled with a known concentration of titrating solution. This is known as the standard solution or titrant.
- Slowly add and mix measured volumes of the standard solution into the solution in the beaker. After each addition, the pH is measured and recorded. This step is repeated until the reaction hits the equivalence point, at which moles of H+ ion from the acid equal moles of OH– ion from the base.
Titration Curve
A titration curve is a graph with the x-coordinate representing the amount of titrant added since the start of the titration and the y-coordinate representing the concentration of the analyte at the corresponding stage of the titration (in an acid-base titration, the y-coordinate usually represents the pH of the solution).
The titration curve in an acid-base titration represents the strength of the matching acid and base. The curve will be somewhat smooth and fairly steep near the equivalence point for a strong acid and a strong base. As a result, a slight change in titrant volume around the equivalence point results in a big pH change, and several indicators (such as litmus, phenolphthalein, or bromothymol blue) are appropriate.
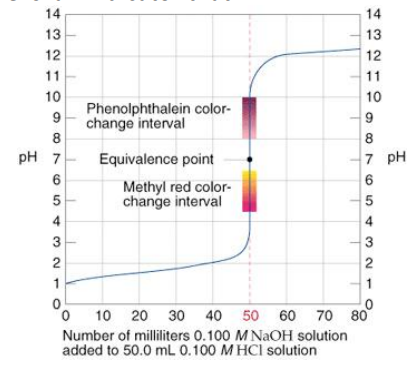
For example: The titration of strong acid by a strong base:
A steep rise in the pH of the acid solution during the titration of a strong acid by a strong base implies that all of the H+ ions from the acid have been neutralized by the OH– ions of the base. The equivalence point of the titration is the point at which the curve flexes. Bromothymol blue is a color indication that changes at this point of equivalency. It’s worth noting that phenolphthalein and methyl red don’t quite meet the equivalence point, but the slope is so steep that it doesn’t matter.
Types of Titrations
Titration of Acid-Base (Acidimetry or Alkalimetry)
Acid-base titrations are primarily based on the neutralization of an acid and a base in solution. More importantly, the strength of an acid is determined by using a standard base solution. This is also known as acidimetry.
Acids are classed as strong or weak based on the degree of dissociation they undergo when dissolved in water. If an acid solution of known concentration is titrated against a strong base, the acid concentration can be estimated after the neutralization reaction has completed. Because of this, only a strong base is employed in the titration procedure. The acid solution is the titrate in this situation, while the strong base is the titrant or standard solution.
Double Titration
In this sort of titration, the titrate (unknown concentration) solution comprises more than one component.
Titration of the mixture against a strong acid is performed to determine the composition of the mixture or to assess the purity of a sample. However, because there will be two endpoints during the titration, two indicators are used instead of one. Indicators such as phenolphthalein and methyl orange are frequently utilized.
Redox Titration
These titrations are quite similar to volumetric acid titrations. Base titrations, with the exception that the reactions involved are Redox reactions. The emphasis here is on determining the unknown concentration of a reducing or oxidizing agent. Titration of oxidizing or reducing agents against strong reducing or oxidizing agents, respectively. In most redox titrations, one of the reactants will act as an indicator (self indicator), changing color according to its oxidizing state.
Precipitation Titration
Precipitation titration is a type of titration in which precipitation forms during the titration procedure.
The titrant reacts with the analyte to generate an insoluble material known as a precipitate in precipitation titration. It will continue until all of the analyte has been consumed. It is a titrimetric approach that involves the generation of precipitates throughout the titration experiment. The analyte reacts with the titrant, forming an insoluble material. The titration process is repeated until the last drop of analyte is consumed. When the titrant reaches its limit, it reacts with the indicator and signals the end of the titration process.
Complexometric Titration or chelatometry
Complexometric Titration, also known as chelatometry, is a sort of volumetric analysis in which the coloured complex is employed to calculate the titration’s endpoint. Titration is a popular procedure used in laboratories to determine the unknown concentration of a previously defined analyte. It is a quantitative chemical analysis approach.
Complexometric Titration is the detection of distinct metal ion combinations in a solution. With each drop of titrant added, the reaction quickly reaches an equilibrium condition. There would be no possibility of any interfering occurrences. A complexometric titration can be used to precisely identify the equivalent point. It is well recognized that EDTA is utilized as a titrant.
Watch this video, you will learn what apparatus needs to be used to conduct a titration, including pipettes, burettes and conical flasks. Titration experiments enable us to work out the exact concentration of an unknown solute, when we know the concentration of another solute. You can calculate the concentration of acids and alkalis through this method.
References
- Vogel, A.I.; J. Mendham (2000). Vogel’s textbook of quantitative chemical analysis (6 ed.). Prentice Hall
- https://byjus.com/jee/titration/
- https://www.vedantu.com/chemistry/volumetric-analysis.
- https://byjus.com/chemistry/acid-base-titration/.
- https://www.vedantu.com/chemistry/complexometric-titration.
- https://teachntest.org/redox-titrations-principle-examples-indicators/.
- https://www.vedantu.com/chemistry/precipitation-titration
- https://webstor.srmist.edu.in/web_assets/srm_mainsite/files/downloads/Precipitation_Titration.pdf


Please upload the practical video