The study of the flow of heat or any other form of energy into or out of a system as it undergoes a physical or chemical transformation is called Thermodynamics. Simply, it is the study of the relationship between heat, work, temperature, and energy. How energy is transferred within a system and whether or not that system can exert useful work on its environment are described by the laws of thermodynamics.
- Zeroth Law of Thermodynamics: Whenever two systems are in thermal equilibrium with a third, they are also in thermal equilibrium with one another.
- First Law of Thermodynamics: The difference between the amount of heat a system absorbs from its environment and the amount of work it performs on its environment is the change in internal energy.
- Second Law of Thermodynamics: The entropy of the universe as a whole, considered as a closed system, can only increase with time. The expansion or contraction of the entropy of the universe can never be negative, according to the second law.
- Third Law of Thermodynamics: According to the third law of thermodynamics, the entropy of a system at zero temperature is a constant.
Interesting Science Videos
Scope of Thermodynamics
- The majority of the key laws of physical chemistry, such as the distribution law, phase rule, and van’t Hoff law of lowering vapour pressure, can be derived from the laws of thermodynamics.
- It indicates if a specific physical or chemical transformation is possible at a specific temperature, pressure, and concentration.
- In determining how far a physical or chemical change can go before the equilibrium conditions are achieved, it is also helpful.
Limitations of Thermodynamics
- Unlike microscopic systems, which are made up of individual atoms or molecules, thermodynamics can be applied to macroscopic systems consisting of matter in bulk. It doesn’t take into account how molecules and atoms are actually formed.
- The second law of thermodynamics disregards the passage of time. Therefore, it cannot be used to determine the rate of a chemical or physical reaction. It cares exclusively about the initial and final states of system.
Thermodynamics Terms and Basic concepts
System, Boundary, and Surrounding
In thermodynamics, a system refers to the region of the universe being studied, whereas the rest of the universe is the surrounding. The boundary is the physical or conceptual line that separates the system from its environment.
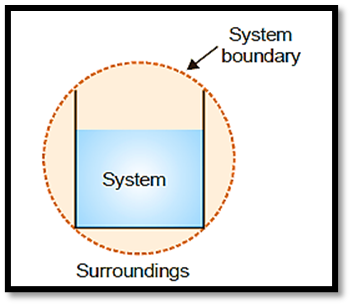
A system consists of a certain amount of one or more chemicals used in an experiment. A beaker containing 200 grams of water is an example of a thermodynamic system. The surrounding consists of the beaker and the surrounding air.
Homogeneous and Heterogeneous system
A system is said to be homogeneous if and only if its components are all the same. Pure single solids, liquids, or gases, gas mixes, and solids that have truly dissolved in liquids are all examples of it. A homogenous system consists of only one distinct phase. A phase is an uniform, discrete, and mechanistically separable part of a system.
A system with more than one phase is said to be heterogeneous. That is to say, it’s not consistent throughout. Ice in contact with water or vapour is an example of a heterogeneous system. The ice, water, and steam all exist as separate phases in this system.
Two intensive properties characterize the thermodynamic state of a homogeneous system. In contrast, a maximum of two state variables may be altered independently. Therefore, there are two thermodynamic degrees of freedom in a homogeneous system. Each phase of a heterogeneous system may be viewed as a homogeneous subsystem, one that is “open” because exchanges with the other phases take place. Therefore, the number of intensive properties that define the thermodynamic state of a heterogeneous system is inversely related to the quantity of phases present. If those characteristics remain independently, a heterogeneous system would have more degrees of freedom. But further restrictions are brought in by thermodynamic equilibrium between phases.
Types of Thermodynamic systems
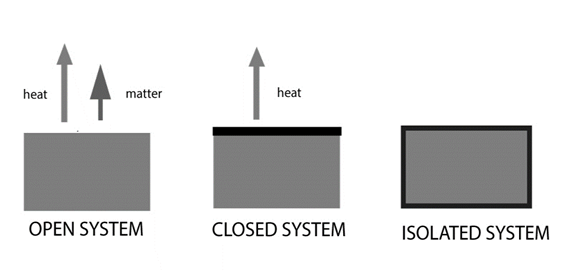
Depending on the type of boundary, there are three different types of thermodynamic systems. No matter can pass through a closed boundary. It is impossible for energy (like heat) to move across an insulated boundary.
Isolated system: No interaction with the environment is possible when the boundary is both sealed and insulated. Therefore, a system is considered isolated when it is unable to exchange energy or matter with its surroundings.
Let’s think about a system 100 cc of water in an enclosed, insulated vessel in contact with its vapour. No water vapour or other material may exit the sealed vessel. Additionally, no heat (energy) can be transferred with the environment because the vessel is insulated. Another illustration of an isolated system is a substance enclosed in a thermos flask, such as boiling water.
Closed system: The boundary in this instance is sealed yet uninsulated. Therefore, a closed system is one that cannot transmit matter but can transfer energy to and from its surroundings in the form of heat, work, and radiation.
An example of a closed system is a certain amount of hot water trapped in a sealed tube. This technique prevents water vapour from escaping, yet it still allows heat to be transferred to the environment through the tube’s walls. A closed system is one in which the gas in a cylinder with a piston is contained. The gas expands as the piston is raised, transferring heat (energy) in the form of work to the environment.
Open system: The border in such a system is open and uninsulated. As a result, an open system is one that may exchange both matter and energy with its surroundings. An open system is a beaker of hot water that is placed on a lab table. Through the imaginary boundary, heat (energy) and water vapor (matter) are transmitted to the surroundings. Another illustration of an open system is the reaction of zinc granules with dilute hydrochloric acid to produce hydrogen gas in a beaker. The heat of the reaction is transmitted to the environment as hydrogen gas escapes.
Intensive and Extensive Properties
The macroscopic or bulk properties of a system (volume, pressure, mass, etc.) can be divided into two classes; Intensive and Extensive properties.
Intensive Properties
A property which does not depend on the quantity of matter present in the system, is known as Intensive Property. Some examples of intensive properties includes pressure, temperature, density, and concentration. Besides these, Temperatures, densities, colors, melting and boiling points, and other characteristics are all intensive properties because they are unaffected by changes in the size or quantity of the material. Since it is an intensive property, the density of one litre of water or one hundred litres of water won’t change.
Since intensive properties are independent of sample quantity and are constant under all situations, they can be utilized to identify samples.
Extensive Properties
A property that does depend on the quantity of matter present in the system, is called an Extensive Property. Volume, moles, enthalpy, entropy, and Gibbs free energy are a few examples of extensive properties. Consider two boxes constructed of the same material, one with a four-liter capacity and the other with a ten-liter capacity. In comparison to a box with a four-litre size, the ten-litre box will hold more material.
In contrast to intensive properties, which are not additive by definition, extensive properties are. The system should be viewed as “a glass of water.” The volume, number of moles, and system internal energy are all increased by doubling the amount of water.
State of system
When the properties of thermodynamic system are all fixed, we say that it is in a particular state. Pressure (P), temperature (T), volume (V), mass, and composition are the fundamental properties that determine the state of a system. These properties are known as State variables, State functions, or Thermodynamic parameters, depending on the context, because a change in their magnitude affects the state of system. It is also reasonable to assume that the state variables will shift as the system transitions from the first to the second state.
All the properties (state variables) need not be stated in order to define a system fully. The chemical makeup of a pure gas cannot be altered because it is always 100% pure. The Equation of State describes the relationship between the three remaining state variables, P, V, and T.
The equation of state, then, for one mole of a pure gas is as follows:
PV = RT
R represents the gas constant. The value of the third (V) state variable is fixed automatically and can be calculated from the equation of state if two of the three (P, V, T) are specified. P and T are examples of independent state variables because they must be specified in order to define the state of the system. The remaining state variable (V) is referred to as the Dependent state variable because its value is determined by both P and T.
When the state of a system is changed, the resulting change in the state variable (or state function) depends on both the initial and final states of the system.
Equilibrium and Non-equilibrium states
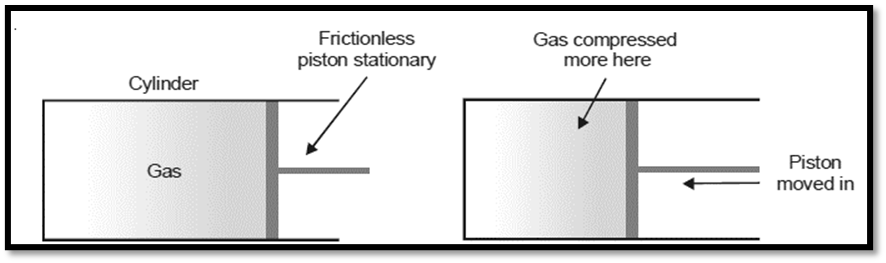
Thermodynamic equilibrium is defined as the condition where all state variables are held constant throughout the system. Let’s pretend there’s a cylinder full of gas with having frictionless piston. When the piston is in a fixed position, the state of gas can be described by its pressure and volume. When that happens, the system is said to be in equilibrium.
When the values of the state variables of system are different in different parts, we say that the system is in a non-equilibrium state.
Since thermodynamics is only concerned with equilibrium states, some of the characteristics of equilibrium states are:
- There can be no temperature gradients or differences between the system and its environment (thermal equilibrium).
- There needs to be mechanical equilibrium, which means that the system’s mechanical properties are consistent everywhere. In other words, no part of the system performs any kind of mechanical work on any other part of the system.
- Without a net chemical change, the chemical composition of system must be stable (chemical equilibrium).
Thermodynamic Processes
A process is any operation that results in a transition from one thermodynamic state to another. In thermodynamic processes, heat energy is transferred either inside or between systems. These processes involve the change of conditions (temperature, pressure and volume). The different processes frequently encountered in thermodynamics are as follows:
- Isothermal processes
- Adiabatic processes
- Isobaric processes
- Isochoric processes
- Cyclic processes
Isothermal Processes: Isothermal processes are those in which the temperature does not change. To do this, the mechanism is typically placed in a thermostat (a constant temperature bath). In simple words, An isothermal process is one in which no heat is added to or lost from the system, such as when hot water is removed from a thermos flask while maintaining the same temperature throughout.
For an isothermal process dT = 0
Adiabatic processes: An adiabatic process is one in which there is no net transfer of heat from the system. Carrying the procedure in an insulated container, such as a ‘thermos’ bottle, may assist to portray adiabatic conditions. Thermal insulation can be achieved through the use of a high vacuum and highly polished surfaces. If the process is exothermic, the heat is absorbed by the system and cannot be released. If the process is endothermic, the system’s own decreased temperature provides the heat that is absorbed.
For an adiabatic process dq = 0
Isobaric Processes: Isobaric processes are those that occur at a constant pressure. Water, for example, boils and evaporates at the same atmospheric pressure when heated to its boiling point. The term “isobaric processes” is used to describe these transformations since they occur at constant pressure.
Let’s pretend there’s fuel in a piston and cylinder setup. The combustion of this fuel causes the engine’s gas pressure to rise. A constant pressure can be maintained, however, if the gases are allowed to expand by moving the piston externally.
For an isobaric process dp = 0
Isochoric Processes: Isochoric processes are those in which the volume does not change during the course of the process. Isochoric processes include those in which a substance is heated without expanding. Isochoric processes are those in which the volume does not change over time. An example of an isochoric process would be the heating of a substance in a non-expanding chamber.
For isochoric processes dV = 0
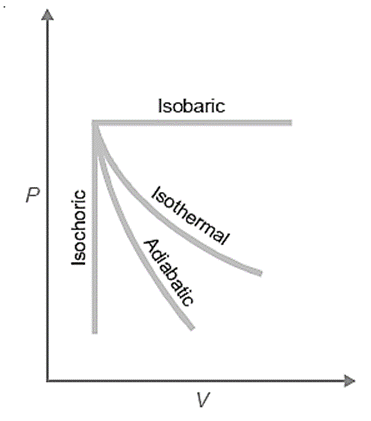
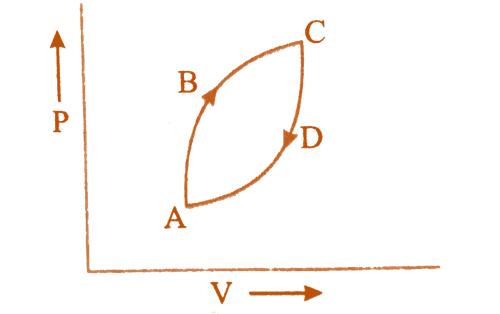
Cyclic Processes: A cycle or cyclic process is a process in which a system in a given state passes through multiple processes before returning to its initial state.
For a cyclic process dE = 0, dH = 0
Reversible and Irreversible Processes
A thermodynamic reverse process is one that moves at an infinitesimally slow rate and can have its direction changed at any time by an equally small shift in the initial conditions of the system. In fact, it is generally agreed that a reversible process involves an unlimited number of extremely little steps that go from the initial condition to the final one. The system is in balance during initial, final, and all intermediate stages. This is because each intermediate step only makes a minor change in the state of system.
An irreversible process is one that changes from an initial condition to a final state without passing through any intermediate states. In this case, the system is in a condition of equilibrium just at the beginning and end.
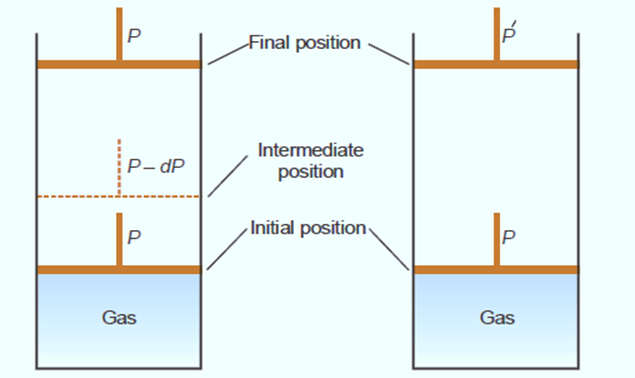
Imagine a cylinder filled with a specific amount of gas and a piston that is both weightless and frictionless. Two approaches, shown in figure, can be used to expand the gas.
The internal pressure of the gas is equal to the pressure P applied to the piston. When the internal pressure is equal to the external pressure, the piston does not move and the volume of the gas does not change. Let’s say now that the piston’s pressure is lowered by a negligible amount, dP. As a result, with an external pressure on the piston of P – dP, the piston will rise and the gas will expand by a negligible amount.
As a result, the gas will expand indefinitely gradually, conforming to the laws of thermodynamics. Since dP is so little over the whole expansion process, the gas remains in equilibrium at all times. The gas undergoes reversible contraction if the pressure is increased by dP at any time during the process. However, if the pressure on the piston is suddenly reduced, the expansion is irreversible. It undergoes a rapid climb in a single motion. Only in the first and last stages does the gas reach a state of equilibrium. In this case, the gas expands in a way that cannot be reversed.
References
- Atkins, P.W. and Julio de Paulo, Atkins’ Physical Chemistry, Oxford University Press, UK, Indian Edition 9, 2011
- R. Chang, “Physical Chemistry for the Chemical and Biological Sciences”, University Science Books, Sausalito, California (2000).
- https://chemistnotes.com/physical/thermodynamic-process-isothermal-isobaric-isochoric-adiabatic-and-cyclic-process/
- https://byjus.com/physics/various-processes-in-a-thermodynamic-system/
- W.S.C. Williams. Nuclear and Particle Physics. Clarendon Press; 1 edition, 1991, ISBN: 978-0198520467
- U.S. Department of Energy, Nuclear Physics and Reactor Theory. DOE Fundamentals Handbook, Volume 1 and 2. January 1993
- Bawendi Moungi G., Alberty Robert A. and Silbey Robert J. (2004). Physical Chemistry. J. Wiley & Sons, Incorporated.
- Alberty Robert A. (2003). Thermodynamics of Biochemical Reactions. Wiley-Interscience.
- Dunning-Davies, Jeremy (1997). Concise Thermodynamics: Principles and Applications. Horwood Publishing. ISBN 978-1-8985-6315-0. OCLC 36025958.
- Kroemer, Herbert & Kittel, Charles (1980). Thermal Physics. W.H. Freeman Company. ISBN 978-0-7167-1088-2. OCLC 32932988
