There is no universal mechanism or explanation for the occurrence of catalytic reactions because they are so diverse. Several hypotheses have frequently been put forward to explain the mechanism of catalysis, but none of them are comprehensive enough to explain all types of catalysis processes. However, two hypotheses of catalytic action have been proposed to explain the phenomenon of catalysis.
The first is an intermediate compound production theory, which postulates the formation of intermediate compounds. The second is known as adsorption theory, which states that reactant molecules adsorb on the surface of the catalyst, giving a lower energy path for the reaction that would not occur in the absence of the catalysts.
Intermediate compound formation theory
According to this hypothesis, a catalyst’s function is to initiate the desired chemical reaction amongst molecules that would not usually have the necessary energy for this to occur. The high energy need is avoided by using a lower reaction path with a lower energy barrier. Generally, this is accomplished by producing an intermediate compound between the catalyst and one of the reactants. This intermediate compound can be generated with less energy than the reaction itself. Being unstable, it breaks down to regenerate the catalyst while simultaneously forming the desired reaction product. Hence, the catalyst that operates by the alternate formation and breakdown of unstable intermediate molecules is used repeatedly.
Let’s say that there are two substances, X and Y, that combine in presence of the catalyst C. The production of the intermediate complex XC and its subsequent reaction with Y is as follows.
X + C → XC
XC + Y → XY + C
Examples:
a. Thermal decomposition of potassium chlorate in the presence of manganese dioxide.
2 KClO3 + 2 MnO2 (catalyst) → 2 KMnO4 + O2 + Cl2
2 KMnO4 → K2MnO4 + MnO2 (regererated catalyst) + O2
K2MnO4 + Cl2 → 2 KCl + MnO2 (regererated catalyst) + O2
Overall reaction: 2 KClO3 + 2 MnO2 → 2 KCl + 3 O2 + 2 MnO2
b. Preparation of sulphuric acid from sulphur dioxide, oxygen, and water, by the lead chamber process uses nitric oxide as a catalyst.
O2 + 2 NO (catalyst) → 2 NO2
2 NO2 + 2 SO2 + 2 H2O → 2 H2SO4.NO (intermediate compound)
2 H2SO4.NO → 2 H2SO4 + 2 NO
Overall reaction : 2 SO2 + 2 H2O + 2 NO → 2 H2SO4 + 2 NO
The theory of intermediate compound formation is supported by the fact that intermediate compounds in numerous catalyzed reactions have been separated from the reaction mixture. For example, nitrosy sulphuric acid in the lead chamber process and chlorine as well as potassium permanganate in the preparation of oxygen by thermal breakdown of potassium chlorate in the presence of manganese dioxide as a catalyst. This theory also explains why the catalysts, even in little quantities, are still effective and have an unaltered mass and chemical composition at the end of the process. However , it fails to explain the action of catalytic poisons and activators.
Adsorption theory
Adsorption theory elucidates the mechanism of heterogeneous catalysis.According to this theory the action of the catalyst is essentially two fold.
a. The law of mass action states that an increase in the frequency of collisions per unit of time will increase the rate of reaction. During the reaction, reactants are adsorbed at the catalyst’s surface, and their high concentration as a consequence of this process increases the frequency of collisions per unit of time. Adsorption is the process by which one material’s atoms or molecules become attached to the surface of another. There are two different types of adsorption. They are:
i. Physical adsorption : The reversible phenomenon involves the weak Van der Waals forces between adsorbate and adsorbent. For instance, the H2 adsorption on the charcoal’s surface.
ii. It is an irreversible phenomenon caused by strong chemical interaction between the adsorbate and the adsorbent. For instance, iron produces iron nitride on the surface when heated with N2 gas at 623 K.
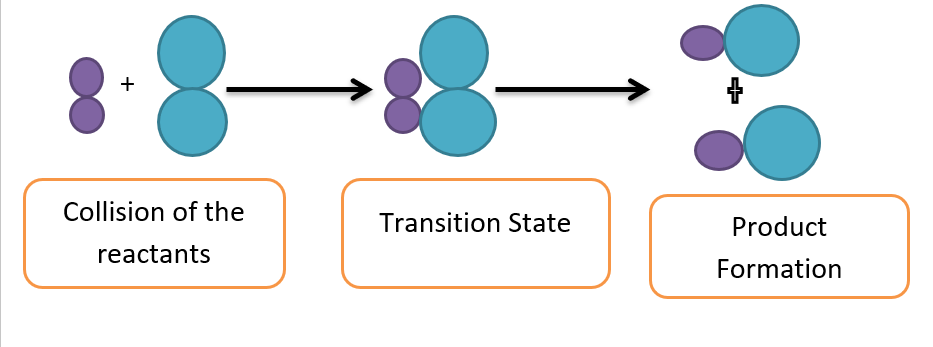
b. The free valencies on the catalyst surface cause chemisorption, which weakens and breaks the bonds between the reactant particles. This gives the reaction a new pathway with a lower energy of activation than the uncatalyzed reaction. As a result, in catalyzed reactions, the energy barrier between the reacting molecules is lower, making it easier to overcome.
Catalysis and activation energy
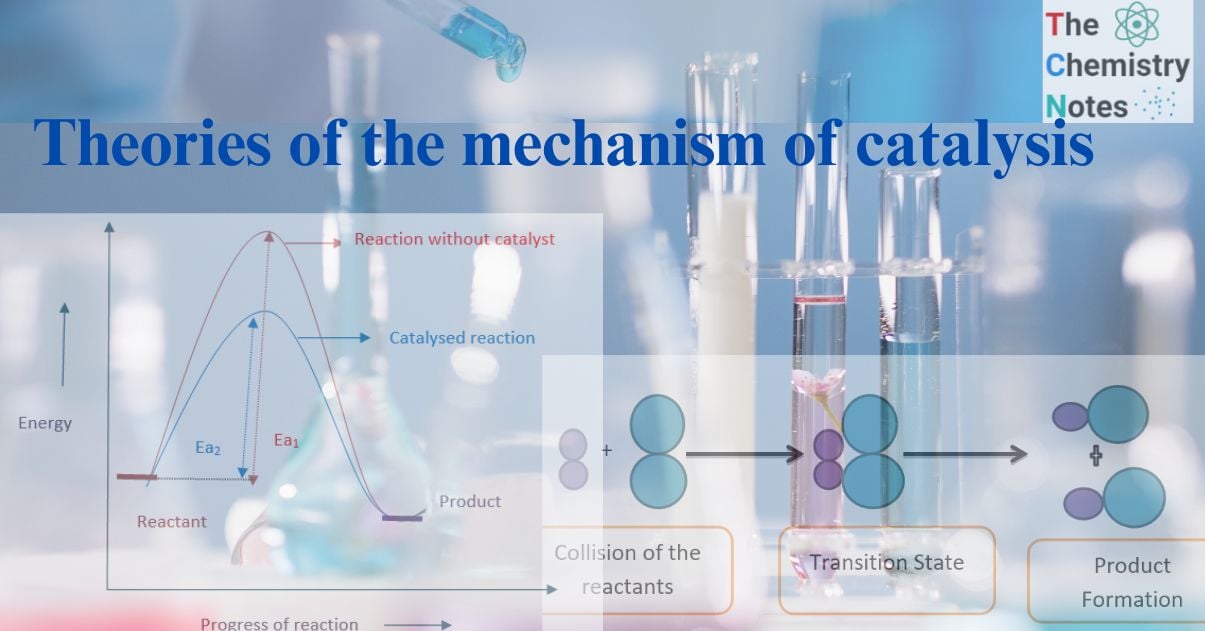
The molecules in the reactants collide to produce a chemical reaction. According to the collision theory, only effective collisions between colliding molecules with sufficient energy and proper orientation cause a chemical change.
At room temperature, the molecules don’t have enough energy, therefore, the collisions are ineffective. The molecules do not react until the minimum quantity of energy (activation energy) is not attained.
Hence, activation energy is the minimum energy required by reacting molecules to initiate a chemical reaction.
When the temperature rises, the molecules’ kinetic energy rises along with their activation energy, which they use to collide to form an activation complex. The decomposition of the activated complex produces the desired products.
Collision between reacting molecule
A catalyst lowers the activation energy by providing a new pathway, causing the reaction to speed up.
Energy profile diagram for catalysed and uncatalysed reaction
Free vacancies and catalyst action
Every atom in a metal’s mass is connected to the atoms around it, and all of its valencies are satisfied. On the other hand, an atom located on the catalyst’s surface has a free valency that is directed outward.
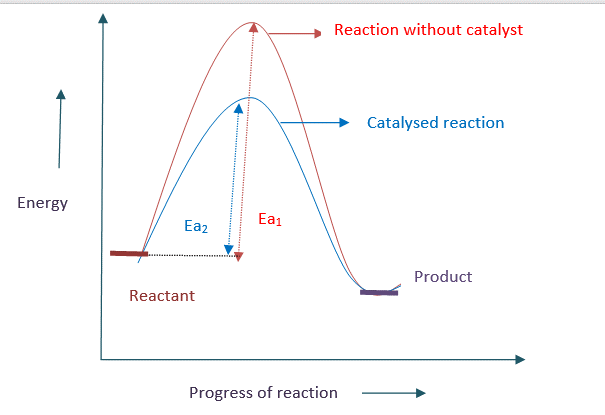
As a result, the surface of the catalyst becomes a site of chemical attraction, similar to the surface tension of a liquid. When the gas comes into contact with the catalyst’s surface, the weak chemical combination binds these molecules to the catalysts.If distinct molecules are adsorbed next to each other, they may react, and the newly produced molecules may evaporate, opening the way for the new reactants molecules. Solid catalysis is used for gaseous processes (contact analysis). For instance, the alkene hydrogenation reaction occurring at the solid Ni or Pt surface.
Mechanism of heterogeneous catalysis
The catalytic action would be amplified if there were more free valencies on the catalyst surface if they were the cause of the catalytic action as previously believed. There are two approaches to improving free valencies:
a. By sub division of the catalyst
b. By rough surface of the catalyst
Sub division of catalyst
This theory states that colloidal or finely powdered catalyst particles, that are considerably richer in free valencies than solid catalysts, should be more effective.
In practical use, colloidal platinum and finely divided nickel are recognized to be the most effective catalysts. This substantially confirms the preceding theory.
Rough surface of the catalyst
The rough surface’s edges, corners, fractures, and peaks have the greatest free valencies and are called active centers. This is mostly responsible for the catalyst’s efficiency.
Effect of temperature on catalysed reaction
Altough, temperature generally increases the pace of a catalyst-mediated reaction, some catalysts are physically altered by high temperature, so their catalytic power may be reduced. This is especially true for colloidal catalysts such as platinum sol, as temperature increases can promote coagulation. In such circumstances, there are two opposing effects
i. increase in reaction velocity with temperature.
ii. A decrease in reaction speed brought on by the catalyst’s partial breakdown at a higher temperature.
Hence, there must be an optimum temperature for the catalyst to have maximum efficiency.
Catalytic poisoning
Heterogeneous catalysts are highly sensitive to the presence of foreign substances, making them ineffective. Catalytic poisoning is a term used to describe any substance that reduces or even destroys the ability of the catalyst to drive the reaction. The catalyst may be poisoned temporarily or permanently. In temporary poisoning, the poison is adsorbed on the catalyst surface rather than the reactants. The activity is immediately restored as rapidly as the molecule leaves the surface’s active region.
In permanent poisoning, the poison and catalyst undergo a chemical reaction, creating a new surface that is catalytically inactive. In such cases, the catalysts can only restart their activity after being recreated in their original form through chemical treatment. Sulfur and arsenic compounds act as poisons to many metallic catalysts.
For example,
a. Traces of arsenic present in the reaction gases render platinum catalytically inert in the contact process for the synthesis of sulphuric acid.
b. Traces of hydrogen cyanide render colloidal platinum useless in catalyzing the breakdown of hydrogen peroxide.
All poison compounds have residual free valencies, which play an essential role in their preferential adsorption on the catalyst surface, effectively rendering the catalytic surface ineffective.
Activation of catalyst
A small number of foreign elements, which may be chemically inert itself, can significantly increase the efficacy of particular catalysts. A promoter or activator is a chemical that, when introduced in relatively small amounts, increases the activity of a catalyst. This process is known as activation.
The promoters enhance the number of active centers by increasing the roughness of the catalyst surface and producing discontinuities in the crystal.
For example, The catalytic activity of iron, which functions as the real catalyst, is markedly increased when several high melting and difficultly fusible oxides, such as aluminum oxide, chromium oxide, rare earth oxide, etc., are added in small amounts during the production of ammonia.
Suggested video:
References
- https://www.brainkart.com/article/Theories-of-Catalysis_41341/#:~:text=The%20action%20of%20catalysis%20in,(ii)%20the%20adsorption%20theory.&text=For%20a%20chemical%20reaction%20to,to%20form%20the%20activated%20complex.
- https://chemistrypage.in/catalysts-theory-and-uses-of-catalysts/.
- https://www.toppr.com/ask/en-np/content/concept/theories-of-catalysis-203387/.
- https://www.thermopedia.com/content/618/.
- https://byjus.com/chemistry/adsorption-theory-heterogeneous-catalyst/.
- https://qsstudy.com/intermediate-compound-theory-of-mechanism-of-catalysis/.
