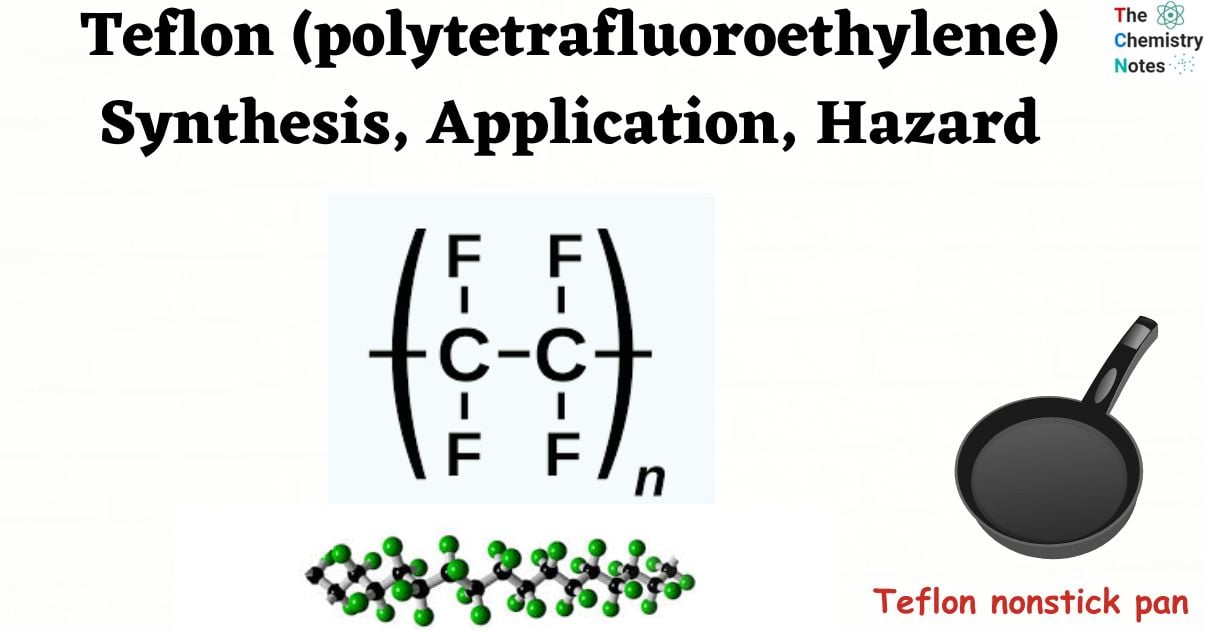
Teflon is a fluoropolymer made of fluorine and carbon atoms, sometimes referred to as PTFE (polytetrafluoroethylene) or polytetrafluoroethylene. It is most famous for being used in non-stick cookware, which is coated on pans to stop food from sticking. The fluorine atoms give the material excellent mechanical, thermal, and electrical qualities and protect the carbon backbone. Low friction, high heat, and chemical resistance are just a few of Teflon’s characteristics. Various uses, such as wire coatings, low-friction coatings, bearings, chemical tank liners, and cookware, are employed in all industries.
What is Teflon?
Tetrafluoroethylene is the monomer used to create the synthetic fluoropolymer Teflon. It is known chemically as poly (1,1,2,2) tetrafluoroethylene. The chemical composition of Teflon is (C2F4)n. Repetitive, or n, numbers of C2F4 units are displayed in the Teflon formula. At low temperatures (about 5K), it can maintain high strength, hardness, and self-lubrication; beyond 194 K, it can maintain good flexibility.
![Structure of Polytetrafluoroethylene [Teflon]](https://scienceinfo.com/wp-content/uploads/2023/05/image-208.png)
Discovery Of Polytetrafluoroethylene
While working for DuPont, an American chemical company, in New Jersey in 1938, Roy J. Plunkett, an American chemist, unintentionally discovered polytetrafluoroethylene. While Plunkett tried to create a new chlorofluorocarbon refrigerant, the tetrafluoroethylene gas in the pressure bottle stopped flowing. He discovered a waxy, white substance that was strangely slippery on the inside of the bottle. Following analysis, it was shown to be polymerized perfluoro ethylene, with the iron from the containers inside catalyzing high pressure.
Synthesis Of Polytetrafluoroethylene
The production of PTFE polymer/resin typically occurs in two steps.
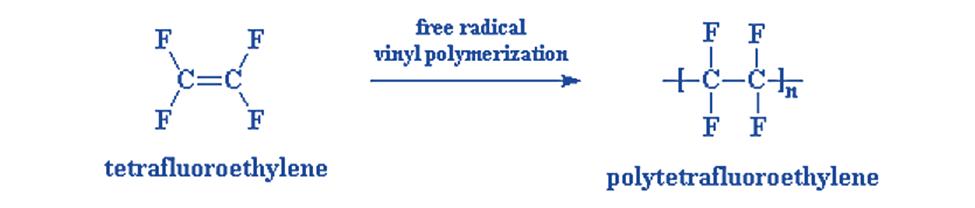
- First, TFE Monomer is often produced by combining Calcium Fluoride (Fluorospar), Sulphuric Acid, and Chloroform. Tetrafluoroethylene (TFE), a monomer, is free-radical polymerized to create Teflon. In the process of polymerization, relatively small molecules, or monomers, are chemically joined to create a chain-like structure or polymer.
- Next, TFE is polymerized under precisely regulated circumstances to create PTFE. The PTFE molecule has exceptional chemical inertness, great heat resistance, and extraordinary electrical insulating capabilities in addition to excellent frictional properties because it contains stable and powerful C-F bonds.
TFE Monomer Preparation
Tetrafluoroethylene was created for the first time in 1933. Fluorspar, sulphuric acid, and chloroform are currently used in commercial synthesis. Hydrofluoric acid is produced when fluorspar (CaF2) and sulphuric acid combine.

Preparation of monochlorodifluoromethane and tetrafluoroethylene (TFE): Monochlorodifluoromethane is produced by reacting hydrofluoric acid with chloroform.

Monochlorodifluoromethane has a boiling point of -40.8°C. In addition, it is utilized as a refrigerant. To prepare the monomer monochlorodifluoromethane pyroleted, for example, by passing through a platinum tube at 700°C.
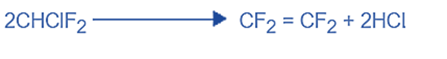
Purification of TFE
Polymerisation requires a pure monomer. Impurities in the final product will have an effect. The gas is cleaned to eliminate any hydrochloric acid before being distilled to separate any remaining contaminants.
TFE polymerization
Pure free Tetrafluoroethylene can polymerize violently, even at temperatures initially lower than room temperature. A silver-plated reactor, quarter-filled with a solution containing 0.2 parts ammonium persulphate, 1.5 parts borax, and 100 parts water, with a pH of 9.2. The reactor was shut down, evacuated, and 30 parts of monomer were added. After one hour of agitation at 80°C, the reactor yielded 86% polymer.
PTFE is commercially produced by two basic processes, the first producing a ‘granular’ polymer and the second producing a dispersion of polymer with much finer particle size and lower molecular weight. A 0.1°% aqueous disuccinic acid peroxide solution was used in one technique of generating the latter. The reactions were carried out at temperatures of up to 90°C.
Properties Of Polytetrafluoroethylene
Here we are going to discuss about the different properties of teflon which allows it to for the variety of applications.
- It is a white, odorless solid that is non-toxic and insoluble in most solvents.
- The density of polytetrafluoroethylene is about 2.2 g/cm3.
- Although it has a melting point of 327 °C, its properties start to decline at temperatures over 260 °C.
- It has the third-lowest coefficient of friction of any known solid substance, ranging from 0.05 to 0.10.
- Polytetrafluoroethylene has a dielectric strength of 60 MV/m.
- It is a good electric insulator even in wet conditions.
- It has a non stick properties in the wide range of temperatures.
- It has high chemical resistance, although it can react with some exceptions. Alkali metals in liquid form, fluorine gas under very high temperatures and pressures, and a few organic halogenated compounds like oxygen difluoride (OF2) and chlorine trifluoride (ClF3) are the exceptions.
Application Of Teflon
Due to the different chemical, physical, and mechanical properties of Teflon, it can be used in a wide variety of industries. Some of the applications of polytetrafluoroethylene are discussed here:
- Used In the Automobile Industry
Teflon is extensively used in the automotive sector due to its strength and resistance to heat, chemicals, and abrasion. Polytetrafluoroethylene enhances the quality of numerous car parts, including axles, ball bearings, chassis, exhaust system, exposed parts on motorcycles and dirt bikes, the exterior of the car, fasteners, gaskets, pistons, seatbelt clips, the underbody, and windshield wipers, in addition to protecting and extending the lifespan of a vehicle.
- Used In Aerospace Industry
The spacecraft may be vulnerable to problems such as faulty communications, defective electronics, and inoperable mechanics caused by frigid temperatures and several dangerous cosmic rays. High-performance fluoropolymers are used to improve spacecraft and satellite conductivity, durability, and dependability, which addresses these problems.
Teflon is a material that makes these initiatives possible, from the improved thermal management of space wear to the wires that help with image transmission from space back to Earth. It is also used in gasoline hoses and tubing to guarantee the safe flow of fuel and other aircraft fluids. It is additionally used as a coating for airplane wings.
- Used In Medical Sector
Teflon provides medical-grade coatings that lessen friction and include antimicrobials to satisfy the sterility demands of medical equipment manufacturers. PTFE coatings are advantageous for medical equipment, including analytical and surgical instruments, autoclave and sterilizing equipment, and pharmaceutical product packaging. PTFE porous design can serve as a matrix for organic cells and tissues. Due to its diameter, porosity, and surface microrelief properties, the organ or tissue can restore lost function by organizing directional growth in the polymer structure.
- Used In Chemical Industry
The manufacturing and industrial sectors receive raw materials from the chemical processing industry. Transporting and storing highly reactive chemicals is one of their difficulties. Equipment such as containers, barrels, pipelines, sacks, and small packages are used for large-scale distribution. Teflon is the ideal material to coat pipes, containers, and hoses that transmit corrosive chemicals since it is chemically resistant.
Polytetrafluoroethylene is made to resist cracking, even in harsh conditions. In addition, the chemicals being transported through it don’t cause it to melt or degrade. Teflon protects several forms of monitoring equipment, including sensors, valves, and process vessels, in addition to ensuring a safe and secure environment for the transfer of chemicals.
- Cookware
Nonstick pans are familiar to everybody who grew up in the modern world. It was one of the first applications of Teflon and has since become one of the most extensively utilized materials in home appliances worldwide. Polytetrafluoroethylene hydrophobic nature makes it an excellent coating for nonstick pans.
- In cosmetic products
Numerous studies have showed that Teflon is a prominent element in a variety of cosmetics and skincare products, including foundation, pressed powder, loose powder, bronzer, blush, eye shadow, mascara, shaving gel, lip balm, and anti-aging lotion. It is commonly utilized in anti-aging and cosmetic products, most likely because it produces a smooth and sleek finish. It is also found in grooming equipment such as curling irons, straighteners, combs, and as a coating on trimmer blades, in addition to cosmetics.
- In constructions
To help minimize stress on steelwork construction, Teflon coatings improve the function of expansion joints, slide bearings, gaskets, and bridge bearings. It can also be used as pipe insulation to prevent thermal bridging. Teflon can even be coated onto building glass which gives it a significantly longer life and makes it easier to clean.
Polytetrafluoroethylene plays an important role in ensuring superior reliability and durability while coping with enormous loads, shocks, and extreme weather, whether for bridges, pipelines, or structures. Screws with Teflon coating repel water and grease and are corrosion resistant.
Teflon coatings increase the function of expansion joints, slide bearings, gaskets, and bridge bearings to assist reduce stress on steelwork construction. It can also be used to avoid thermal bridging in pipes. It can even be coated onto building glass, extending its life and making it easier to clean.
Teflon Hazards
Teflon is a non-hazardous and long-lasting material in general cases.
- However, at temperatures exceeding 500°F (260°C), Teflon coatings on nonstick cookware begin to degrade, releasing harmful compounds into the air.
Inhaling these gases can cause polymer fume fever, popularly known as the Teflon sickness. - Polymer fume fever is characterized by flu-like symptoms such as chills, fever, headache, and body aches. The start occurs 4-10 hours after exposure, and the illness normally resolves between 12-48 hours.
- A small number of case studies have also identified more serious side effects of overheated Teflon exposure, including pulmonary damage.
Individuals were exposed to vapors from overheated Teflon cookware at severe temperatures of at least 730°F (390°C) for extended periods of time in all recorded cases.
How to Minimize Your Risk when using Teflon?
There are numerous alternatives to Teflon pots and pans, but if they are not available, it is critical to follow safety requirements. The following are some things you can do to reduce the harm Teflon can do you.
- Cooking over high heat should be avoided.
Due to the harmful fumes emitted by Teflon cookware when heated to high temperatures, keep to low to medium heat and avoid using Teflon under a broiler. - Preheating Empty Cookware
Empty pots and pans can achieve high temperatures much faster than full pots and pans, producing the odors you’re attempting to avoid with less vigorous cooking. - Make use of ventilation.
When cooking with Teflon, make sure to keep fumes to a minimum by using exhaust fans or opening windows. - Avoid Using Metal Utensils
Metal utensils will scratch the cookware, reducing its lifespan. Instead, use utensils made of silicon, wood, or plastic. - Cookware should be washed by hand.
Wash the pots and pans carefully, and avoid using abrasive cleaning sponges such as steel wool or scouring pads.
Frequently Asked Questions (FAQ)
Is Teflon safe?
Experts generally agree that Teflon is not an issue in and of itself. The coating is non-toxic in and of itself. Even if you eat a few flakes of it, it will pass right through you. However, some specialists are concerned about what happens when Teflon becomes overheated. “When pans are overheated, the PTFE coating begins to disintegrate,”
How to use Teflon tape?
Start the tape on the second thread and apply clockwise. This will help to prevent cross threading and guarantee that the tape does not wind up inside the pipe. Once you’ve got the right positioning, keep the tape tight and wrap three times more.
A normal pipe fitting will seal correctly after you thread it in approximately a half-inch or so, depending on the size. As a result, the most typical thread tape is half an inch wide. Keep this in mind while applying the appropriate amount of plumber’s tape and use it as a starting point for how far to thread in your pipe fittings.
Video on Teflon
References
- https://collegedunia.com/exams/polytetrafluoroethylene-teflon-chemistry-articleid-1347
- Ellis, D.A.; Mabury, S.A.; Martin, J.W.; Muir, D.C.G.; Mabury, S.A.; Martin, J.W.; Muir, D.C.G. (2001). “Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment”. Nature. 412 (6844): 321–324. doi:10.1038/35085548. PMID 11460160.
- https://byjus.com/chemistry/teflon/
- https://www.toppr.com/guides/chemistry/chemistry-in-everyday-life/teflon/
- https://www.vedantu.com/chemistry/teflon
- https://orioncoat.com/blog/how-is-ptfe-teflon-made/https://engineeringinterviewquestions.com/chemistry-notes-on-teflon/
- https://www.britannica.com/science/polytetrafluoroethylene
- https://studiousguy.com/uses-of-teflon/

