Sulfur is multivalent non-metal, abundant, and odorless. Pure sulfur is a tasteless, odorless, brittle, pale yellow solid that is a poor conductor of electricity and insoluble in water. It can be found in nature as a pure element or as sulfide and sulfate minerals. Although sulfur is notorious for its odor, which has been compared to rotten eggs, that odor is a characteristic of hydrogen sulfide (H2S).
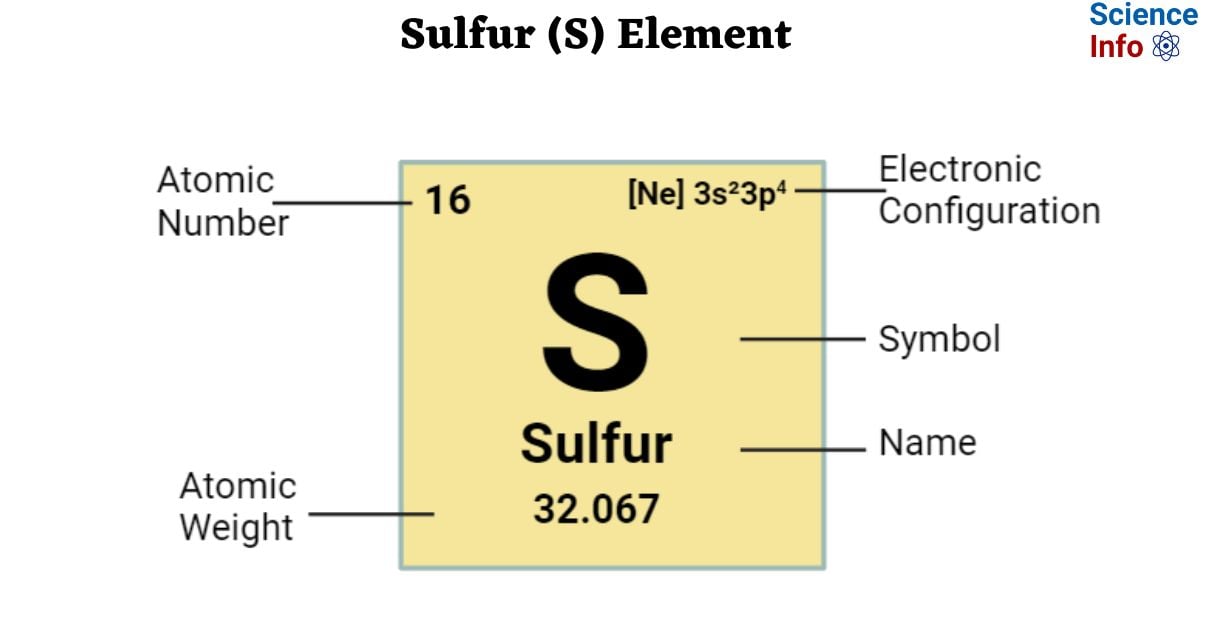
The Symbol of the element Sulfur is S.
Sulfur (S) is a critical element that should never be overlooked. Sulfur (S), also spelled sulphur, is a nonmetallic chemical element of the oxygen group (Group 16 [VIa] of the periodic table), and one of the most reactive. It is a nonmetal and is obtained as a byproduct of natural gas production.
It forms sulfides with all metals except gold and platinum, and it also forms compounds with several nonmetallic elements. Every year, millions of tons of sulfur are produced, primarily for the production of sulfuric acid, which is widely used in industry.
Interesting Science Videos
Element Sulfur History
Sulfur has a long history dating back to ancient times. It was discovered by the Chinese around 2000 BCE. Sulfur has been used in ancient Greece, Egypt, China, and India since prehistoric times. In ancient times, sulfur was known as Torah, and it was also mentioned in the Bible as “brimstone,” which means “burning sulfur.” In Egypt and Greece, sulfur was known for its bactericidal activity and was used for fumigation as well as in medicines and ointments. Sulfur was known as Shilin Huang in China as early as the 6th century BC and was extracted from pyrite. Pre-Roman civilizations used burned brimstone as a medicine and used “bricks” of sulfur as fumigants, bleaching agents, and incense in religious rites. The Chinese primarily used it in black gunpowder. In 1777, Antoine Lavoisier proposed that sulfur is a distinct element but elemental sulfur was discovered in 1867 and its elemental nature was established by the French chemist’s Joseph Gay- Lussac and Louis Thenard.
Element Sulfur Occurrence
Sulfur is abundant both on Earth and throughout the universe. It ranks tenth in abundance among all elements in the universe. Sulfur is formed in massive stars and is found in various types of meteorites. Its formation is mainly during the fusion reaction of helium nuclei and silicon nuclei. Sulfur is the fifth most abundant element in the Earth’s crust in terms of mass. It is common in volcanic regions and hot, dry areas around the world. The Pacific Ring of Fire is particularly well-known for its abundant sulfur reserves. Sulfur is also found in its natural state on Earth, where it is formed by the metabolic activity of anaerobic bacteria that degrade sulfate minerals. Gypsum, pyrite, barite, and other sulfur minerals are the most common.
Sulfur in minerals
galena (lead sulfide, PbS)
blende (zinc sulfide, ZnS)
pyrite (iron disulfide, FeS2)
chalcopyrite (copper iron sulfide, CuFeS2)
gypsum (calcium sulfate dihydrate, CaSO4∙2H2O)
barite (barium sulfate, BaSO4).
Sulfur is currently produced from natural gas, petroleum, and fossil reserves. China and Canada are the two largest sulfur producers. Sulfur is a necessary component of all living cells and is in proteins as well, DNA, and a wide range of enzymes found in plants, animals, and microbes. The human body comprises of various forms and compounds of sulfur, and it is the eighth most abundant element by weight.
Isotopes of Sulfur
Sulfur has four stable isotopes:
32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%).
Allotropes of Sulfur
Sulfur has many allotropes among them the following are of more importance
The monoclinic sulfur (α-sulphur) and yellow rhombic sulfur (β-sulphur). The most intriguing feature is their thermal stability; the sulfur allotropes are interconvertible, i.e. rhombic sulfur when heated above 369K yields monoclinic sulfur.
Elemental Properties of Sulfur
| Electronic Configuration | [Ne] 3s²3p4 |
| Atomic Number | 16 |
| Atomic Weight | 32.067 |
| Group, Period, and Block | 16, 3, p-block |
| Atomic Radius | 180 pm (Van der Waals) |
| Covalent Radius | 105 pm |
| Electronegativity | 2.58 (Pauling scale) |
Physical Properties of Sulfur
- Sulfur is responsible for the formation of many polyatomic molecules. One of the most common types of molecules associated with sulphur is octasulfur.
- It is odorless and bright yellow, and it exists as a soft solid.
- The melting point of sulphur is approximately 115.21° C, and its boiling point is approximately 444.6° C.
- The sulphur molecule polymerizes when it is present between the boiling and melting temperatures, resulting in lower density but higher viscosity.
- Depolymerization occurs at higher temperatures, resulting in decreased viscosity.
- Sulphur has a density of about 2g/cm3, which can vary depending on the allotrope.
- Pure sulphur is a poor conductor of electricity and insoluble in water.
- It forms sulfides with all metals except gold and platinum, and it also forms compounds with several nonmetallic elements.
Chemical Properties of Sulfur
- Sulfur combustion produces a blue flame and an unpleasant odor due to the formation of sulfur dioxide.
- Sulfur is insoluble in water but slightly soluble in nonpolar organic solvents such as benzene.
- This element’s first ionization energy is 999.6KJ/mol, and its second is 2252 KJ/mol. The most common oxidation states are +4 and +6.
- Except for noble gases, sulfur is highly reactive and almost reacts with all elements, including iridium (an unreactive metal).
- Sulfur compounds have many unusual properties, including the ability to catenate like carbon. Sulfur’s properties allow it to form chain structures as well as a ring system, similar to carbon. One of the most well-known sulfur compounds is hydrogen sulfide (H2S).
Reactions of Sulfur
| Chemical Reactions | Description |
| S (s) + H2SO4 (l) → 3 SO2 (g) + 2 H2O (l) | Sulphur does not react with dilute non-oxidizing acids, under normal conditions. Sulfur reacts with hot concentrated sulfuric acid, forming SO2. |
S22− (aq) + 2 H+ (aq) → H2S (g) + S (s) | Disulfide ions react with acid-forming hydrogen sulfide and free sulphur |
| S (s) + O2 (g) → SO2 (g) | Sulphr reacts with oxygen forming sulfur dioxide and trioxide |
S8 (s) + 12 KOH (aq)  4 K2S (aq) + 2 K2S2O3 (aq) + 6 H2O (l) 4 K2S (aq) + 2 K2S2O3 (aq) + 6 H2O (l) | Sulphur reacts with hot aqueous potassium hydroxide, KOH, forming potassium sulfide and thiosulfate |
| C (s) + 2S (s) → CS2 (g) | Carbon reacts with sulfur at high temperatures, in the absence of oxygen, forming carbon disulfide |
S8 (s) + 8 H2 (g)  8 H2S (g) 8 H2S (g) | Sulfur reacts with hydrogen, forming hydrogen sulfide |
| Cd (g) + S8 (g) → CdS (g) Cd (s) + S8 (s) → CdS(s) [yellow] | In gaseous form, Cd and S8 reacts forming CdS. At 130-180 °C solid Cd and S8 reacts explosively, also forming CdS |
| S (s) + 3 F2 (g) → SF6 (s) | Sulfur reacts with excess fluorine forming sulfur(VI)fluoride |
Uses and Application of Sulfur
- Sulfur is easily available in nature as this is in plentiful amounts. Occurring naturally, sulphur has a yellowish color and is in crystal form. At room temperature, sulphur is non-reactive.
- Sulfur is important in the body and is also essential for the synthesis of certain proteins. Sulphur, for example, is crucial component for the synthesis of glutathione, which acts as a powerful antioxidant to protect your cells from damage.
- While sulphur found in food is beneficial to the body, there is little evidence that taking sulphur supplements is beneficial.
- Sulfur is an FDA-approved ingredient for use in over-the-counter dandruff products. It is frequently used in conjunction with salicylic acid. However, there is limited evidence to support that use.
- Sulfur supplements are frequently in use to treat osteoarthritis. MSM may be beneficial to those suffering from knee osteoarthritis.
- Sulfur is necessary for the production of other essential chemicals. Sulphuric acid, the most important chemical produced by sulphur, has numerous industrial applications.
- The reaction of sulphur with methane produces carbon disulfide, which is essential for the production of rayon and cellophane.
- Another important application of sulphur is rubber vulcanization.
- Sulfur compounds, particularly organo-sulfurs, are widely applicable in the pharmaceutical industry. Sulfa drugs are a large class of broad-spectrum antibacterial sulfonamides. Sulphur is also found in penicillin and cephalosporin.
- Since ancient times, people have used elemental sulphur as pesticides and fungicides. Dusting sulphur (powdered sulphur) is a common pesticide in organic farming.
- It is possible that using sulphur topically is safe. In clinical studies lasting up to eight weeks, researchers used products containing sulphur at concentrations of up to 10% safely.
Health Effects of Sulfur
Sulfur is required by all living things. It is especially important for humans because it is a component of the amino acid methionine, which is a must-have in our diet. Sulphur is also found in the amino acid cysteine. The average person consumes approximately 900 mg of sulphur per day, primarily in the form of protein.
Although elemental sulphur is not toxic, many simple sulphur derivates, such as sulphur dioxide (SO2) and hydrogen sulfide, are.
Sulphur is commonly found in nature as sulphides. Several processes add sulphur bonds to the environment, which are harmful to both animals and humans. These harmful sulphur bonds are also formed in nature during various reactions, most notably when substances not naturally present are already present.
Sulphuric substances in the environment can have the following effects on human health:
- Neurological effects and behavioral modifications
- Blood circulation disruption – Heart damage
- Eye and vision effects – Reproductive failure
- Immune system damage – Stomach and gastrointestinal disorders
- Impairment of liver and kidney functions
- Defects in hearing
- Disruption of hormonal metabolism
- Dermatological consequences
- Asphyxia and lung embolism
Environmental Effects of Sulfur
- Sulfur is in a variety of forms in the atmosphere. When animals inhale sulphur in its gaseous state, it can cause eye and throat irritation. Because of the limited possibilities for destroying the sulphur bonds , sulphur is widely applicable in industries and emitted into the atmosphere.
- Sulfur harmful effects on animals are primarily brain damage as a result of hypothalamic dysfunction and nervous system damage.
- Sulfur in laboratory tests on animals reveals to cause serious vascular damage in veins of the brain, heart, and kidneys.
- Certain forms of sulfur have also tests to cause foetal damage and congenital effects. Sulfur poisoning can even be passed on to children by mothers through mother milk.
- Finally, sulfur can harm animals’ internal enzyme systems.
Toxicity, Safety, and Precautions related to Sulfur
Toxicity classification by WHMIS 1988
Emergency Overview: Contains a pressurized gas that if heated may explode but will not catch fire. TOXIC AT ALL LEVELS. If inhaled, it is fatal.
Toxicity
If inhaled it is HIGHLY TOXIC, can result in death. It can cause severe nose and throat irritation. At high concentrations, it can cause a potentially fatal buildup of fluid in the lungs (pulmonary edema). Coughing, shortness of breath, difficulty breathing, and chest tightness are some of the symptoms. A single high concentration exposure can result in a long-term condition such as asthma. Many things, such as other chemicals or cold temperatures, can easily irritate the airways if this happens.
Contact with the skin: CORROSIVE. The gas causes Skin irritation or burns. Scarring can be permanent. Direct contact with the liquefied gas can cause the skin to chill or freeze (frostbite). Numbness, prickling, and tingling are all symptoms of mild frostbite. The skin may develop a waxy white or yellow appearance. In severe cases, blistering, tissue death, and infection may occur.
CORROSIVE eye contacts: The gas causes irritation or burns in the eyes. Permanent damage, including blindness, is possible. Direct contact with the liquefied gas can cause the eye to freeze. Blindness or permanent eye damage may result.
Long-Term Effects (Chronic) Exposure: May cause respiratory problems. It has the potential to irritate and inflame the airways.
Safety Measures
- Before attempting a rescue, take precautions to ensure your safety (e.g. wear appropriate protective equipment). Get the victim some fresh air. If breathing becomes difficult, emergency oxygen should be administered by trained personnel. Allow the victim to move around unnecessarily.The onset of pulmonary edema symptoms may be delayed. Call a Poison Control Center or a doctor right away.
- In the case of contact with the skin, flush for 5 minutes with lukewarm, gently flowing water. DO NOT RUB the area or apply direct heat to it. Remove any clothing or jewelry that may be impeding circulation. Remove the rest of the garment after carefully cutting around clothing that sticks to the skin. Cover the affected area loosely with a sterile dressing. DO NOT PERMIT THE VICTIM TO DRINK OR SMOKE. Call a Poison Control Center or a doctor right away.
- Move the victim to fresh air if they make eye contact. Flush the contaminated eye(s) immediately with lukewarm, gently flowing water for 5 minutes while keeping the eyelid(s) open. Avoid direct contact with liquefied gas. If necessary, use chemical protective gloves. Flush with lukewarm, gently flowing water right away. . Apply a sterile dressing to both eyes.
Precautions
- In the event of a spill or leak, put on an escape-type respirator and leave the area immediately. Report any leaks, spills, or safety equipment failures immediately (e.g. ventilation system). Maintain the cylinder in an upright position. Keep cylinders safe from harm. To move cylinders, use a suitable hand truck; do not drag, roll, slide, or drop. When not in use or empty, keep containers tightly closed. Use tools and equipment that are resistant to corrosion .
- Keep cool, dry, well-ventilated, temperature-controlled, away from incompatible materials, out of direct sunlight, and away from heat and ignition sources. Only authorized personnel should have access. In the event of a leak or spill, keep escape-type respiratory protective equipment on hand.
For immediate help:
Centers for Disease Control and Prevention Public Response Hotline (1-888-246-2675)
Agency for Toxic Substances and Disease Registry (1-888-422-8737)
Regional Poison Control Center (1-800-222-1222)
Note: Please do not get confused with Sulfur and Sulphur. Both kinds of spellings are used within the content.
References
- Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- Egon Wiberg; Nils Wiberg (2001). Inorganic Chemistry. Academic Press. pp. 513–. ISBN 978-0-12-352651-9.
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/sulfur-isotopes
- https://www.ccohs.ca/oshanswers/chemicals/chem_profiles/sulfurdi.html
- https://pilgaardelements.com/Sulfur/Reactions.htm
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_16%3A_The_Oxygen_Family_(The_Chalcogens)/Z016_Chemistry_of_Sulfur_(Z16)
- https://www.webelements.com/sulfur/chemistry.html
