Transition metal complexes, like organic compounds, exhibit stereoisomerism, specifically geometric and optical isomerism. Geometric isomers, on the other hand, are only possible for square planar and octahedral complexes, not tetrahedral. In contrast, optical isomers are only possible for tetrahedral and octahedral complexes, not square planar.
Depending on the coordination number, transition metal complexes can have a variety of shapes. Tetrahedral, square planar, and octahedral are common shapes for transition metal complexes formed with monodentate ligands (ligands that only form one bond to the central metal ion or atom). The octahedral shape of 6-coordinated complex ions is due to the central metal being attached to six ligands. 4-coordinated complex ions, on the other hand, in which the central metal is attached to four ligands, can have either a tetrahedral or square planar shape. The bond angle differs between these two shapes: in tetrahedral structures, the bond angle is 109.5 degrees, whereas, in square planar structures, the bond angle is 90 degrees.
Geometrical (cis-trans) isomerism
Transition element complexes are still capable of having geometric isomers despite not having a double bond.
Cis-trans isomerism is observed in square planar and octahedral complexes with two pairs of different ligands.
Cis-platin, an anti-cancer drug, is an example of a square planar complex with two pairs of ligands.
Trans-platin, unlike cis-platin, cannot be used in cancer treatment because it binds to DNA in cancer cells.
As long as a complex ion has two ligands that are distinct from the others, the complex can exhibit geometric isomerism. Geometrical isomers are metal complexes that differ only in whether their ligands are adjacent to one another (cis) or directly across from one another (trans) in the coordination sphere of the metal. They are especially significant for square planar and octahedral complexes.
Square planar complexes
Complexes with four-coordinate bonds may occasionally adopt a square planar geometry rather than a tetrahedral one. Because all square vertices are equivalent, it makes no difference which vertex in a square planar MA3B complex is occupied by the ligand B; thus, only a single geometrical isomer is possible in this case (and in the analogous MAB3 case). Because they can be superimposed simply by rotating the complex in space, all four structures shown here are chemically identical.
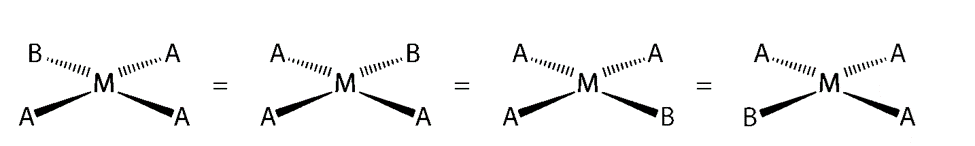
There are two possible isomers for a MA2B2 complex: either the A ligands are adjacent to each other (cis), in which case the B ligands must also be cis, or the A ligands are across from each other (trans), in which case the B ligands must also be trans. Despite the fact that the cis isomer can be drawn in four different ways and the trans isomer in two different ways, all members of each set are chemically equivalent:
![square planar complex [cis-isomer]](https://scienceinfo.com/wp-content/uploads/2022/11/image-1.png)
![square planar complex [trans-isomer] Stereoisomerism in transition metal complexes](https://scienceinfo.com/wp-content/uploads/2022/11/image-2.png)
They are fundamentally different arrangements of atoms in space because there is no way to convert the cis structure to the trans structure by rotating or flipping the molecule in space.
Octahedral Complexes
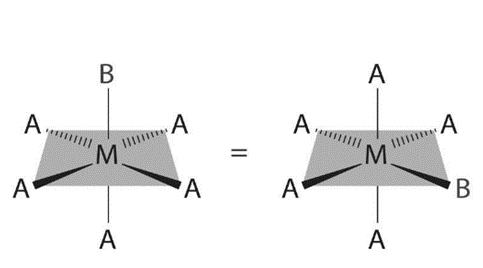
There are also cis and trans isomers in octahedral complexes. Like square planar complexes, octahedral complexes have only one structure possible because only one ligand is different from the other five (MA5B). Despite the fact that we usually draw an octahedron in such a way that the four “in-plane” ligands are distinct from the two “axial” ligands, all six vertices of an octahedron are equivalent. As a result, any MA5B structure can be superimposed on any other representation simply by rotating the molecule in space. The following are two of the many possible orientations of an MA5B structure:
Two isomers are possible if two ligands in an octahedral complex differ from the other four, resulting in a MA4B2 complex. The two B ligands can be trans or cis. This type of system includes cis- and trans- [Co (NH3)4Cl2] Cl:
![Octahedral complex [cis-isomer]
Stereoisomerism in transition metal complexes](https://scienceinfo.com/wp-content/uploads/2022/11/image-4.png)
![octahedral complex [trans-isomer] Stereoisomerism in transition metal complexes](https://scienceinfo.com/wp-content/uploads/2022/11/image-5.png)
Optical Isomerism
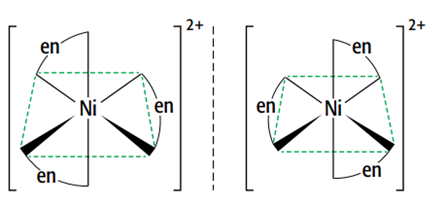
There are optical isomers of octahedral complexes with bidentate ligands.
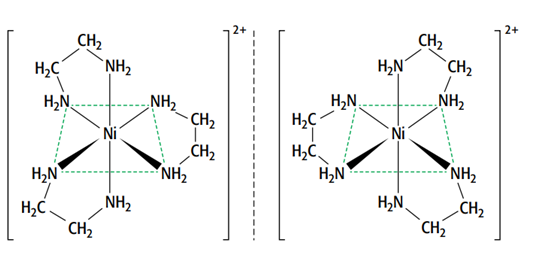
This means that the two forms are non-superimposable mirror images of each other. They have no plane of symmetry, and one image cannot be placed directly on top of the other. The only difference between the optical isomers is their ability to rotate the plane of polarized light in opposite directions.
[Ni(H2NCH2CH2NH2)3]2+ and [Ni(H2NCH2CH2NH2)2(H2O)2] 2+ are two octahedral complexes with optical isomers.
Video on Stereoisomerism in transition metal complexes
References
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/6-inorganic-chemistry-a-level-only/6-3-transition-element-complexes-isomers-reactions–stability-a-level-only/6-3-5-stereoisomerism-in-transition-element-complexes/
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Coordination_Chemistry/Structure_and_Nomenclature_of_Coordination_Compounds/Isomers/Stereoisomers%3A_Geometric_Isomers_in_cis-platin
- https://blogs.ntu.edu.sg/cy1101-1819s1-g09/2018/10/stereochemistry-in-transition-metal-complexes/#:~:text=Apart%20from%20organic%20compounds%2C%20transition,but%20not%20tetrahedral%20%5B2%5D.
- Lancashire, R.J. Isomerism in Coordination Compounds 2015: http://wwwchem.uwimona.edu.jm/courses/IC10Kiso.html

