Steam distillation is a type that is employed particularly for materials sensitive to high temperatures. It is a method that separates non-volatile contaminants from high-boiling compounds with the help of steam.
It is a specific type of distillation utilized for temperature-sensitive materials such as natural aromatic compounds. It was previously a popular laboratory technique for the purification of organic substances. It entails distilling at lower temperatures that reduce the degradation of the intended products.
The term “steam distillation” is employed for a batch of continuous distillation using open steam. The liquid is distilled by directly injecting open steam into the distillation still, where the steam carries the vapors of the volatile liquid component and is subsequently condensed to separate the liquid from water.
Steam distillation is achievable only when the following conditions are met:
- The substance does not react with steam at the provided temperature and pressure.
- The substance is insoluble in water.
Principle of steam distillation
When a mixture of two nearly immiscible liquids is heated, the liquid exerts its vapor pressure as a function of temperature as if the other constituent were not there.
As the temperature rises, the entire system’s vapor pressure also increases. Boiling begins when combined vapor pressures of the two immiscible liquids exceed the atmospheric pressure.
This allows for the purification of numerous organic molecules that are insoluble in water at temperatures much below the temperature at which decomposition occurs.
Bromobenzene, for instance, has a boiling point of 156 0C, whereas water has a boiling point of 100 0C. However, a mixture of the two boils at 95 0C. Thus, bromobenzene can be readily distilled at 61 0C below its typical boiling point.
Working process of steam distillation
Most organic molecules are complex and do not dissolve in water. However, they produce a mixture that can be separated if it is allowed to settle down. During this process, the organic molecules float to the top when the water settles down.
According to the steam distillation method’s principle, the system’s vapor pressure will rise when a mixture of two or more immiscible liquids is heated due to the combined vapor pressure of two immiscible liquids. This permits components with high boiling points to evaporate at even lower temperatures by allowing them to form a mixture with water.
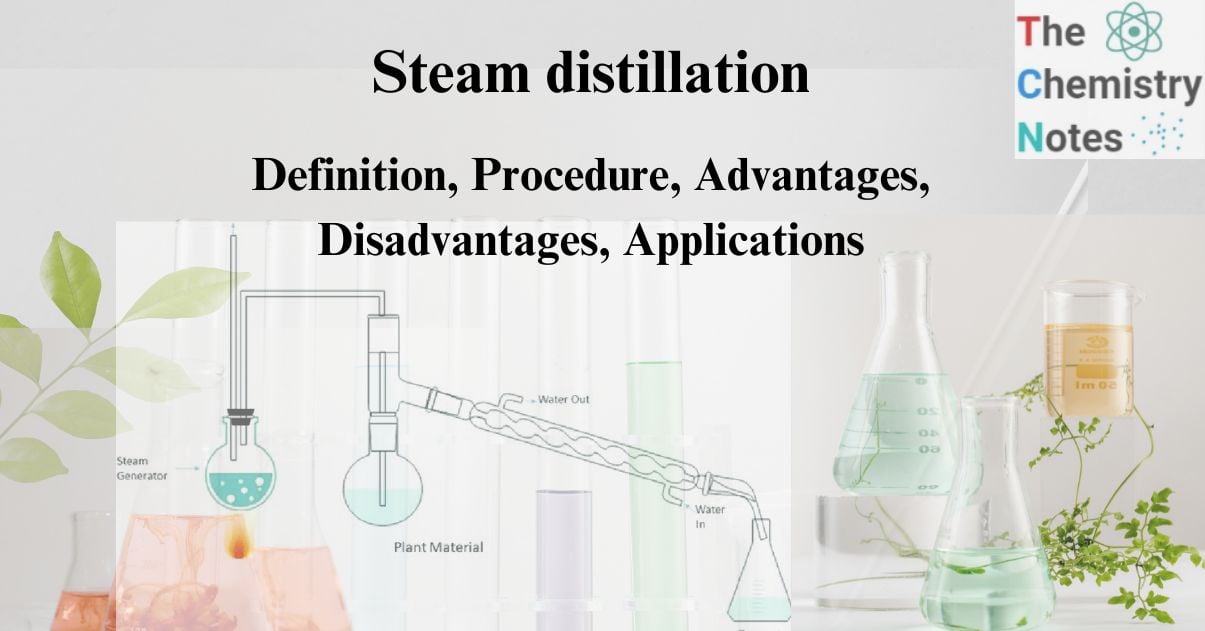
Extraction procedure of steam distillation
In this extraction technique, steam passes through the organic matter and is condensed, resulting in a mixture of the steam and the substance. The additional stem is now heated after passing through the entire mixture. This causes the mixture to evaporate. Due to the lower vapor pressure, the required organic components evaporate as part of the mixture. So organic materials are extracted from the mixture.
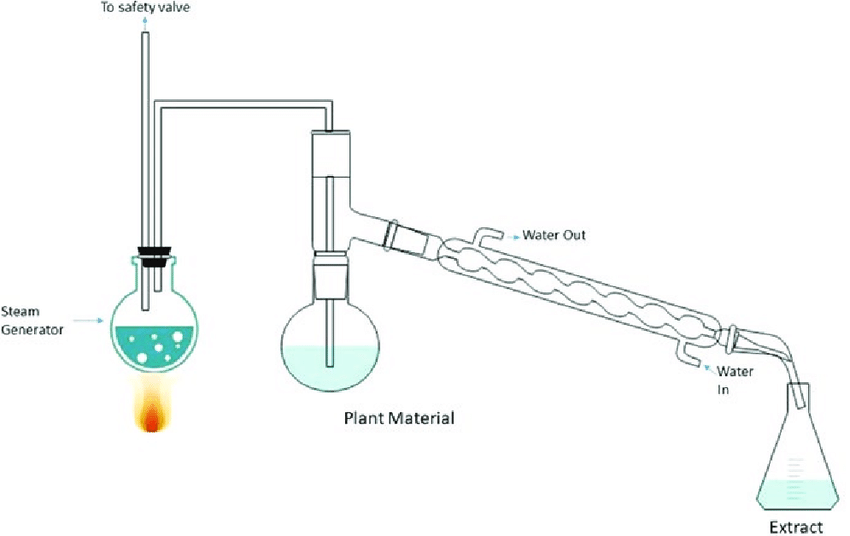
Extraction process by steam distillation
image source: https://www.researchgate.net/publication/333664831_Essential_Oils_from_Pines_Chemistry_and_Applications
Separation procedure of steam distillation
The evaporated mixture of steam and organic molecules flows through the container, with cold water entering from one end. After passing through the cold water, the evaporated mixture also consists of cold water. The mixture is now fed through the hot water from the other end. As a result, the mixture condenses.
The mixture is collected and settled for separation. The extracted organic chemicals appear at the top. They are then separated by filtering the water from below.
Extraction of essential oil by steam distillation
The distinctive smell of many naturally existing plants is caused by volatile oils, which exist in low concentrations in these plants. These essential oils contribute to the distinct odor of numerous plants, including eucalyptus, citronella, garlic, oranges, roses, peppermint, and many more.
Unfortunately, when heated to higher degrees, many of these oils decompose.
In general, steam distillation is employed to extract essential oils because steam distillation uses low-pressure steam to replace volatile compounds in plant material. Aside from that, steam distillation aids in controlling the temperature and the amount of steam applied to the plant material during the extraction process.
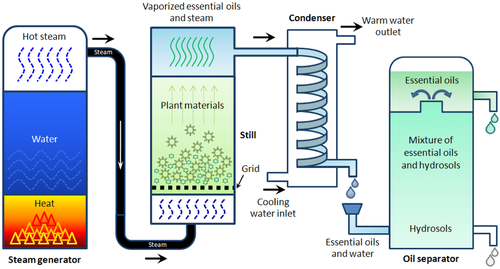
Extraction of essential oil by using steam distillation
Image source: https://ift.onlinelibrary.wiley.com/doi/full/10.1111/1750-3841.12492
In this extraction technique, an equipment chamber with a hole in the bottom is present, and this hole allows steam to travel through either fresh or dried herbs. A lid is also provided in the chamber, preventing the oil from spreading into the air when steam is supplied to it. The oil and water droplets can then be collected. Water and oil are thus separated by passing them through the filter.
Physiochemical reactions involved in the steam distillation process
Almost all of the steam distillation process involves three major physiochemical reactions. These procedures outline the benefits and drawbacks of this specific process. By considering the example of a plant cell containing essential oil, these physiochemical reactions are described below:
I) Diffusion
When steam comes into contact with plant cells, the osmosis process begins. When the system reaches the boiling point of water, this process begins. The osmosis process occurs in this oil-water solution after a portion of the volatile oil dissolves in the water available inside the glands containing the essential oils. It eventually makes its way to the surface, where the oil is evaporated by passing steam. Before beginning the method, the substance should be combined and distilled with water for optimum oil output.
II) Hydrolysis
The hydrolysis process begins after the diffusion process. Hydrolysis refers to the reaction of water with specific elements of essential oils in this essential oil extraction technique. Esters, for example, react with water at high temperatures to generate acids and alcohol. This byproduct reduces the amount of the intended ingredient, essential oil, produced.
This hydrolysis process produces more water while simultaneously producing more byproducts. This causes the processing time to increase. This technique has the significant disadvantage of steam distillation. As a result, we should utilize the least amount of water possible.
III) Decomposition by heat
At higher temperatures, most essential oil components become unstable. To avoid the degradation of the compounds by heat, the distillation process must be carried out at lower temperatures.
Factors affecting steam distillation
I) Water
Water is one of the elements that influence the steam distillation process. More water increases by-products and processing time. As a result, a smaller amount of water should be utilized.
II) Temperature
The rate of crude oil steam distillation is directly related to temperature. The distillation rate of crude oil gradually increases as the steam temperature rises. However, the highest temperature can degrade the essential component.
III) Viscosity
The rate of steam distillation is inversely related to the viscosity. As viscosity increases, the rate of steam distillation decreases.
IV) Pressure
The rate of steam distillation is inversely proportional to pressure. The distillation rate reduces as the system pressure rises.
Advantages of steam distillation
a. Quality control
The steam distillation technique allows us to control the temperature and amount of steam passed through the plant material. The degree of degradation of the components will be reduced after maintaining the temperature at the boiling point of water. So we can readily get the intended compound.
b. Separation of compound
The steam distillation method is used to separate temperature-sensitive organic molecules such as aromatic compounds. It also aids in extracting oils from natural products such as citrus oil, eucalyptus oil, and other natural substances obtained from organic matter.
c. Removing the impurities
Steam distillation is useful for removing the impurities from a compound. This is because the boiling point of a compound is related to its purity. The higher the purity of a compound, the higher its boiling point. Thus, the compounds with a high purity will vaporize at a higher temperature than those with low purity.
d. Extraction of essential oil
Steam distillation is a technique for extracting and isolating essential oils from plants for use in natural products. Steam is injected through the plant in the container where the aromatic molecules of the plant are released and converted into vapor.
e. Reduces the decomposition of temperature-sensitive compounds
Steam distillation helps in the reduction of the decomposition of temperature-sensitive compounds by lowering the boiling point. It is useful for the purification of organic compounds.
Disadvantages of steam distillation
- The liquid mixture with comparable boiling points cannot be separated since both evaporate at the same temperature and are converted to vapor. So, we cannot collect them separately.
- It needs high equipment and operating cost.
- Special training is required to operate such an instrument.
Applications of steam distillation
The Steam Distillation technique is utilized in numerous fields to carry out various industrial activities. Some of these applications are as follows:
a. Extraction of essential oil
Essential oils are highly volatile chemicals extracted by several physical procedures from a single botanical species’ plant. Steam distillation is commonly employed for this purpose on both a small and large scale.
b. Separation of fatty acids from mixtures
Steam distillation is carried out for the purification of the fatty acids. It is used in the process of determination of a certain amount of fatty acids in a certain compound.
c. Checking the quality of food materials
Steam distillation process is used in various industrial processes in order to check the quality and impurity levels in certain foods.
d. Petroleum refineries
Crude oil contains various components and each of these components is isolated in a stepwise procedure in oil refineries. This process is carried down by using steam distillation procedures.
e. Juice analysis
The amount of volatile acids contained in certain juices and wines is estimated by using the steam distillation procedures.
f. Fragrances and flavors
Steam distillation is carried out in the production of fragrances and flavors for the food and beverage industry.
suggested video:
References
- https://kau.in/sites/default/files/announcements/11_sample_chapter_1707_steam_alhilphy.pdf.
- https://www.mdpi.com/2071-1050/14/12/7119.
- https://byjus.com/chemistry/steam-distillation/.
- https://courseware.cutm.ac.in/wp-content/uploads/2020/05/Steam-Distillation.pdf.
- https://www.vedantu.com/chemistry/steam-distillation.
- https://www.researchgate.net/publication/285643201_Extraction_Steam_Distillation.
- https://www.ethosnatura.com/what-is-steam-distillation-of-essential-oils/.

Is just as efficient to boil two strongly agitated immiscable liquids compared to injecting steam through the mixture?