The scientific study of how various types of matter emit and absorb radiation, including light, is known as spectroscopy. They deal with the radiation’s wavelength. Another subject that spectroscopy studies is the interactions between protons, electrons, and ions. Spectroscopy can also investigate particle interactions and collision energy. Quantum mechanics, relativity, and quantum electrodynamics use spectroscopy.
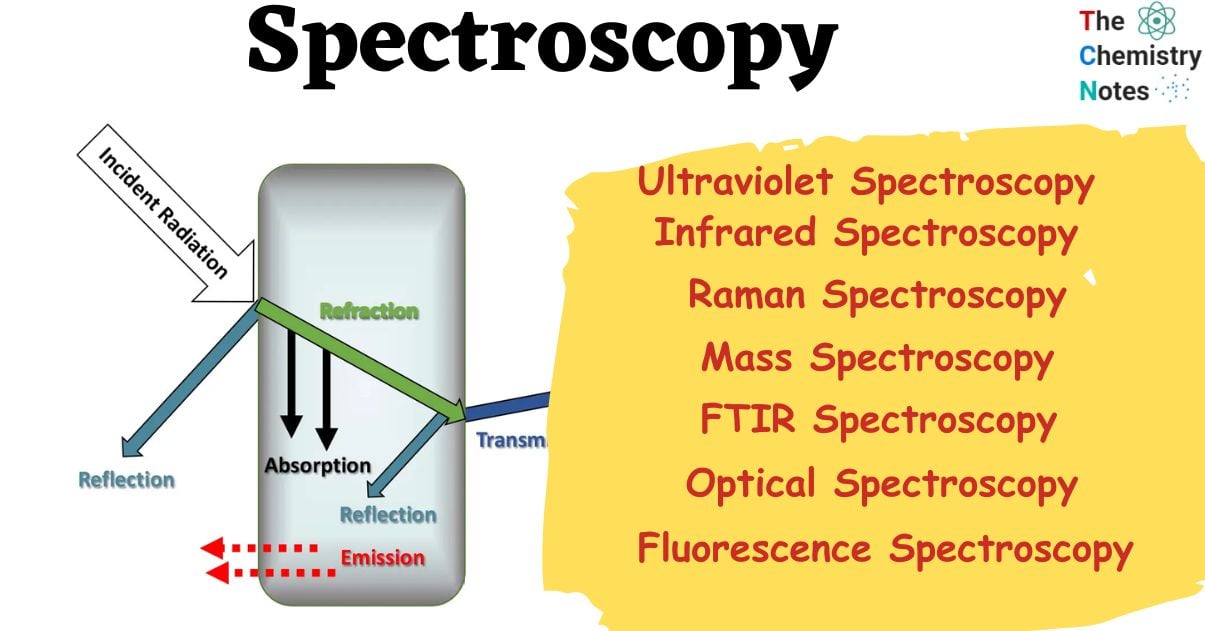
The primary purposes of spectroscopy are to recognize and clarify the components of atoms and molecules. They are measured by looking at the sample’s or object’s radiant energy discharges or absorptions. Here, a beam of electromagnetic radiation like infrared or UV rays is passed onto the sample, and the response is measured through the wavelength of the electromagnetic spectrum applied from the external energy source.
Interesting Science Videos
What is Spectroscopy?
Spectroscopy is a field of study that investigates the interaction between matter, such as molecules, atoms, and nuclei, and radiated energy, specifically electromagnetic radiation. Consequently, this field of study pertains to the examination of the interactions between molecules, compounds, atoms, or the nucleus and various electromagnetic radiations mentioned above.
The categorization of spectroscopy depends upon the manner in which matter interacts with radiation.
- Absorption: The process of absorption takes place when the matter in question fully assimilates the electromagnetic radiation that it encounters. Various matter species, including atoms and molecules, exhibit selective absorption of distinct forms of electromagnetic radiation. The quantification of absorption is commonly achieved through the observation of the quantity of radiation that passes through the substance.
Two frequently used forms of spectroscopy that involve absorption are ultraviolet-visible spectroscopy (UV-Vis spectroscopy) and infrared spectroscopy (IR spectroscopy).
- Emission: It refers to the process by which matter emits electromagnetic radiation. The occurrence of emission can be initiated by an exogenous energy source, such as flames (in the context of flame photometry), or by electromagnetic radiation of greater energy (in the context of fluorescence).
Fluorescence spectroscopy typically involves the observation of emission.
- Scattering, diffraction, and reflection: This spectroscopic technique relies on how the electromagnetic radiation of interest is dispersed, diffracted, or reflected. X-ray crystallography is an instance of scattering or diffraction, wherein high-energy X-rays are utilized to investigate the atomic arrangement of proteins and molecules in crystal form.
- Resonance or Coherence: The spectroscopic technique of Resonance or Coherence involves the utilization of electromagnetic radiation to establish a coherent interaction with the quantum states of matter.
Nuclear Magnetic Resonance spectroscopy (NMR spectroscopy) is an instance of this category of spectroscopy.
- Inelastic Scattering: The occurrence of inelastic scattering is noted when the incident radiation’s wavelength undergoes a shift after its interaction with matter.
Raman spectroscopy provides an instance of inelastic scattering.
Spectrometer
A spectrometer is a type of scientific instrument. It is primarily used to measure and separate the spectral components of electromagnetic radiation according to their physical properties and to assess the wavelength of the radiation. For molecular spectroscopy, the spectrometer is frequently employed. The radiation source, detection, and analysis tools make up the spectrometer. When molecules in a sample are excited to higher energy states by emission spectrometers, the radiation they produce when they revert to lower energy states is examined.
Types of Spectroscopy
The types of spectroscopy and their properties and applications are discussed below:
Ultraviolet Spectroscopy
UV spectroscopy is also known as absorption spectroscopy or reflectance spectroscopy. The electromagnetic spectrum of the ultraviolet region lies adjacent to that of the infrared region. UV spectroscopy is mainly used for bacterial culture, drug identification, and checking nucleic acid purity.
Infrared Spectroscopy
Infrared spectroscopy mainly deals with the electromagnetic spectrum in the infrared region. They work on absorption spectroscopy. IR spectroscopy is used to identify the chemical composition of the material. Fourier transform infrared (FTIR) spectrometers mainly use IR spectroscopy techniques. The electromagnetic spectrum of infrared is classified into three types: near-infrared, far-infrared, and mid-infrared.
Near-infrared: The near-infrared ranges between 14000-4000 cm-1, which helps to study overtones or harmonic vibrations.
Mid-infrared: The mid-infrared ranges from 4000-400 cm-1, which will help to study the fundamental vibrations and associated rotational-vibrational structure.
Far-infrared: The far-infrared ranges from 400-10 cm-1, which helps to study microwave regions with low energy and may be used for rotational spectroscopy.
Raman Spectroscopy
Photon absorption is the foundation of Raman spectroscopy. It will evaluate the substance based on whether photons are scattered at a higher or lower frequency. Depending on the oscillation or rotation of the molecules, photons may gain or lose energy when they strike molecules or atoms.
Rayleigh scattering is the scattering process where the sample disperses the bulk of the incident photons without changing their frequency. The monochromatic visible laser will often have a Raman spectrum. The radiation is analyzed using a detector that consists of a scanning optical monochromator and a phototube.
Mass Spectroscopy
Mass spectrometry, or mass spectroscopy, is an analytical tool for measuring the mass-to-charge ratio (m/z) of one or more molecules in a sample. It is used to study protein-protein interactions. These measurements can often be used to calculate the exact molecular weight of the sample components as well. Every mass spectrometer consists of at least these three components:
Ionization Source: For external electric and magnetic fields to move and manipulate molecules, they must first transform them into gas-phase ions. In the laboratory, a technique called nanoelectrospray ionization is used, similar to how cars are painted industrially. This method allows the creation of positively or negatively charged ions, depending on the experimental requirements. The output of a small-scale chromatography column and the input of a mass spectrometer can be directly coupled using nano-electrospray ionization.
Mass Analyzer: Once ionized, the ions are sorted and separated according to mass-to-charge (m/z) ratios. There are various mass analyzers on the market right now, with trade-offs like operation speed, separation resolution, and other operational requirements. The mass analyzer often works in concert with the ion detection system.
Ion Detection System: Following separation, the ions are measured and transferred to a data system where the m/z ratios and relative abundances are stored. Simply plotting the m/z ratios of the ions in a sample against their intensities produces a mass spectrum. The heights of the peaks indicate the relative abundance of the different components, and each peak in a mass spectrum represents a component with a specific m/z in the sample.
FTIR Spectroscopy
FTIR spectroscopy is also known as Fourier-transform infrared spectroscopy. This technique is obtained by obtaining an infrared spectrum of the absorption or emission of a solid, liquid, or gas.The radiation that passes through the sample is recorded. Because different molecules with different structures produce different spectra, the spectra can be used to identify and distinguish among molecules. It is widely used for analyzing nano and biological materials, water content determination in plastics and compositions, detectors in chromatography, etc.
Optical Spectroscopy
Optical spectroscopy is the study of how matter interacts with electromagnetic radiation. It is frequently used in the pharmaceutical industry and can determine a sample’s metal content, the concentration of an active ingredient, the sample’s color, or its identity. Optical spectroscopy utilizes a wide spectral range of 0.2 nanometers to 500 microns.
Fluorescence Spectroscopy
Fluorescence spectroscopy is one type of electromagnetic spectroscopy. They are mainly used for the fluorescence of a sample. Usually, UV lights are used in fluorescence spectroscopy for analyzing organic components in biochemical, medical, and chemical research fields. By using microfluorimetry, it can be adopted at the microscopic level. Using atomic fluorescence spectroscopy (AFS) techniques, we can find the compound present in air, water, or other media.
Uses Of Spectroscopy
- Spectroscopy is mostly used to figure out how molecules and atoms are put together. The structure and electron configurations of atoms and molecules are examined using a wavelength.
- Spectroscopy can also be used to determine the chemical makeup of materials that are unknown. Focusing on a few parts per million of a trace element will be made easier thanks to its emission spectrum.
- The study of spectral emission lines will help astronomers study distant galaxies. This will help to analyze the universe in all directions. Astronomers also use the Doppler shift of spectral lines for observations. Usually, a Doppler shift will occur when the source of radiation, like stars or nebulae, moves relative to an observer.
- Environmental scientists have employed visible and ultraviolet spectroscopic techniques for a considerable duration.
- The utilization of Raman spectroscopy enables the acquisition of the vibrational spectrum of a specific analyte, commonly known as its distinctive “fingerprint”. This subsequently facilitates the process of identification and interpretation. The potential applications of this phenomenon span a wide range of fields, including archaeology and modern nanotechnology.
References
- Analytical Chemistry: An Introduction (Saunders Golden Sunburst Series) 7th Ed., by Douglas A. Skoog, Donald M. West, F. James Holler. 1999.
- Fundamentals of Analytical Chemistry 8th Ed., by Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch. 2003.
- Helmenstine, Anne Marie, Ph.D. “Spectroscopy Definition.” ThoughtCo, Feb. 16, 2021, thoughtco.com/definition-of-spectroscopy-605676.
- https://www.britannica.com/science/spectroscopy/Techniques-for-obtaining-Doppler-free-spectra
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Bruice)/13%3A_Mass_Spectrometry_Infrared_Spectroscopy_and_Ultraviolet_Visible_Spectroscopy/13.07%3A_Spectroscopy_and_the_Electromagnetic_Spectrum
- https://www.pasco.com/products/guides/what-is-spectroscopy
- https://byjus.com/chemistry/spectroscopy/
