In chemistry, the term “solution” refers to a homogeneous mixture of two or more substances in relative amounts. Although the term “solution” refers to the liquid state of matter, solutions of gases and solids are also possible.
Interesting Science Videos
What is the Solution?
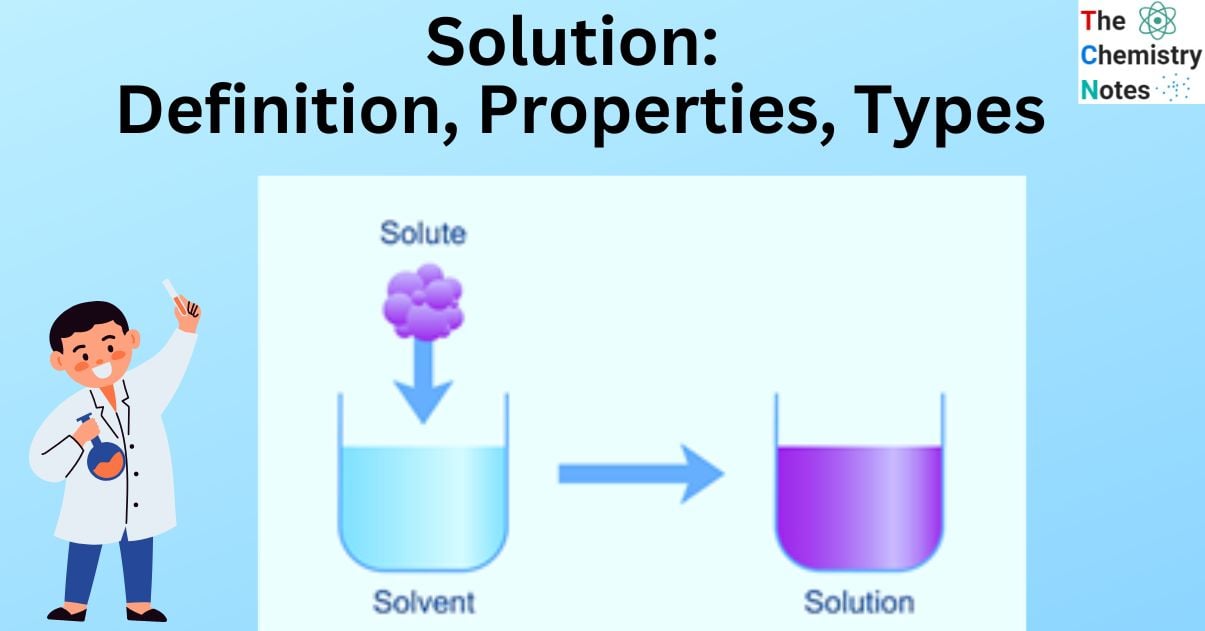
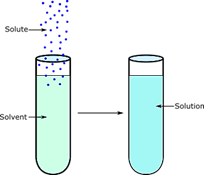
A solution is a type of homogeneous mixture made up of two or more substances, one of which (the solute) is dissolved in another (the solvent). Solids, liquids, and gases can all be present in solutions, which are homogeneous mixtures.
As a result of the homogeneity of the mixture, each sample of a solution is identical in terms of appearance and concentration. Although liquid and gaseous solutions are the most commonly encountered, a solution can exist in any phase.
A solution is made up of two parts: a solute and a solvent. Solutes are substances that dissolve in solvents. A solvent is a medium that dissolves the solute. The macroscopic properties of solutions do not vary across the sample.
The amount of solute that can dissolve in a solvent is referred to as its solubility.

Characteristics of Solution
All solutions have the following characteristics:
- It is a mixture of two or more substances that is homogeneous.
- Solutions are combinations of different solutes and a solvent.
- Filtration cannot separate the constituents of a solute.
- Saturated, unsaturated, and supersaturated solutions are all possible.
- This is stable; solutes will not precipitate unless added in excess of the solubility of the mixture, at which point the excess will remain in its solid phase, a process known as hyper saturation.
- The particles of solute in a solution are invisible to the naked eye. Particles, on the other hand, may be visible in a suspension.
- A solution does not cause light beams to scatter. The particles in a suspension, on the other hand, can cause Tyndall or Rayleigh scattering.
- Heat is often absorbed or evolved during the formation of solutions. When Ca(OH)2 is dissolved in water, for example, heat is released, whereas heat is absorbed when NH4Cl is dissolved in water.
What is Solute?
A solute is defined as a substance that dissolves in a solution.
The solvent is present in greater quantities than the solute in fluid. The concentration of a chemical solution is a measurement of the amount of solute present in relation to the amount of solvent.
Examples of Solutes
Salt in water is a common example of a solute. The solute salt dissolves in the solvent water to form a saline solution.
Water vapor, on the other hand, is considered a solute in the air because nitrogen and oxygen are present in much higher concentrations in the gas.
What is a Solvent?
A solvent is a substance that dissolves a solute to form a solution.
The solvent is the most abundant component in the solution and determines the physicochemical form of the substance as solid, liquid, or gas. Solvents are typically liquids, but they can also be gases or solids.
Solvents can be primarily acidic, primarily basic, amphoteric (both) or aprotic (neither). The polarity of the solvent particle is critical in determining the solute’s solubility in the solvent.
Examples of Solvent
Aromatic compounds and other hydrocarbons, alcohols, esters, ethers, ketones, amines, and nitrated and halogenated hydrocarbons are examples of organic compounds used as solvents.
Water is a polar compound that is also regarded as a universal solvent capable of dissolving a large number of solute particles.
Difference Between Solute and Solvent
Examples of Solutions
| Nine Solution Type | Example |
| Gas in gas | Gas-gas solutions are solutions in which both the solute and the solvent are gases. For example, 1. nitrogen and oxygen, 2. carbon dioxide and nitrogen, 3. carbon dioxide and oxygen (oxygen and carbon dioxide are solutes; nitrogen is the solvent) |
| Gas in liquid | The term “Gas-Liquid Solutions” refers to mixtures in which the solute is in a gaseous state and the solvent is liquid. For example, 1. oxygen (mixture) in water, and carbon dioxide (mixture) in water. Carbon dioxide in water is a less straightforward example because it is accompanied by a chemical reaction (formation of ions). The visible bubbles in carbonated water are not the dissolved gas, but rather an effervescence of carbon 2. Coca-Cola is an example of a gas-liquid solution because it contains carbon dioxide dissolved in water dioxide that has escaped from the solution; the dissolved gas is not visible because it is dissolved at the molecular level. |
| Gas in solid | Gas-solid solutions are those in which the solvent is in a solid state and the solute is in a gaseous state. For example, 1. Consider a hydrogen solution in palladium. Hydrogen dissolves fairly well in metals, particularly palladium; this is being investigated as a method of hydrogen storage. |
| Liquid in liquid | Liquid-liquid solutions contain both a solute and a solvent in a liquid state. Two or more substances of the same chemistry but different concentrations are mixed together. (Solution homogenization) For example, 1. Alcoholic beverages are essentially ethanol solutions in water 2. The vinegar solution is a mixture of ethanoic acid and water. |
| Liquid in Gas | It contains liquid solute and a gas solvent. For Example; 1. Aerosol, Water vapor in gas |
| Liquid in solid | Liquid-solid solutions contain a solid solvent and a liquid solute. For example, 1. Mercury in gold, forming an amalgam 2. Water dissolved in solid salt or sugar, resulting in moist solids 3. Hexane dissolved in paraffin wax 4. Plasticizer-containing polymers, such as phthalate (liquid) in PVC (solid) |
| Solid in solid | These are ones that contain both the solvent and the solute in a solid state. For example, 1. Gold and copper solution 2. Bronze and a variety of other alloys 3. Barium sulfate and radium sulfate combined to form a solid solution of Ra in BaSO4. |
| Solid in liquid | Solid-liquid solutions are solutions in which the solutes are solid and the solvent is liquid. For example, 1. Sucrose (table sugar) dissolved in water (sugar is the solute; water is the solvent) 2. Table salt, sodium chloride (NaCl), or any other salt that dissolves in water to create an electrolyte. When salt dissolves, it dissociates into ions. |
| Solid in Gas | For Example: Sublimation of substances such as iodine, camphor, and others into the atmosphere. |
Types of Solutions
Based on how much solute they contain; chemical solutions can be divided into different categories:
Dilute: The amount of solvent is much greater than the amount of solute.
Concentrated: It has close to the maximum solute concentration that will dissolve in the solvent.
Saturated: This is a concentrated solution with the greatest amount of dissolved solute based on solubility.
Supersaturated: It contains more solute than the maximum amount of solute that will dissolve in the solvent. This is typically created by saturating a solution at a higher temperature and then slowly cooling it to a lower temperature.
Here is a video with plentiful explanation and examples related to solubility and explain the concept of how compounds dissolve in water. https://youtu.be/MDHlaTHbEgM
References
- Lew, Kristi (2009). “Homogeneous.” Acids and Bases, Essential Chemistry. New York: Chelsea House Publishing. Online publisher: Science Online. Facts On File, Inc. ISBN 978-0-7910-9783-0.
- https://edu.rsc.org/cpd/mixtures-and-solutions/3008735.article
- Brown, T. L. (2009). Chemistry: The Central Science. Pearson Education.
- https://www.thoughtco.com/definition-of-solution-604650
- Kosower, E.M. (1969) “An introduction to Physical Organic Chemistry” Wiley: New York, p. 293

