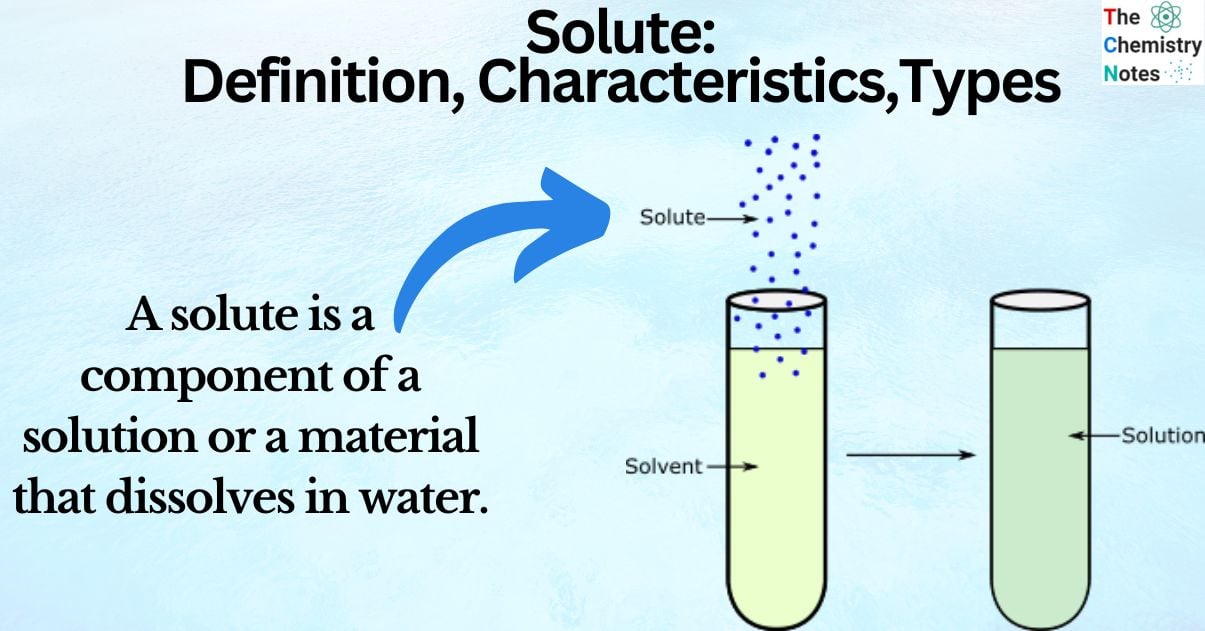
A solute is a substance that dissolves in a solution. In a fluid, the solvent is more abundant than the solute. A chemical solution’s concentration is determined by comparing the amount of solute to the amount of solvent. A typical example is salt in water.
Interesting Science Videos
What is Solute?
A solute is a component of a solution or a material that dissolves in water. It’s concentration in solution is assessed by how much solute is dissolved inside the solvent in relation to how much of the particular solvent is present.
Characteristics of Solute
- Filtration cannot separate the solute from a solution
- It consists of only one phase.
- Three different states solid, liquid, and gaseous.
- The boiling point is higher than solvents.
- The qualities of the solute affect a solution’s solubility.
- When heat is present in a solution, it is transferred to it.
- The solute’s attributes, such as its surface area and molecular size, as well as its solubility define the it’s physical characteristics.
- The substance in the solution that is most abundant is called the solvent.
Types of Solutes
They come in a wide variety of forms, such as salts, sugars, and organic compounds.
- Salts are ionic substances that separate into positive and negative ions in a solvent.
- Sugars are organic compounds that break down in the water to create syrup-like solutions.
- Organic molecules are carbon-containing compounds that easily dissolve in water, alcohol, and oils.
Examples
Salt in Water
A solution is produced when a spoonful of salt is added to a glass of water. The salt, or NaCl, is the solute. H2O, or water, is the solvent. The oxygen atoms are positively charged, while the hydrogen atoms are negatively charged, by the water molecules. Two ions, Na+ and Cl–, make up the ionic compound known as salt. Positive hydrogen atoms draw positive sodium (Na+) whereas negative chlorine atoms are drawn to positive oxygen atoms (Cl–).
It is physically separated from other molecules by attraction, which equally disperses it throughout the water. The amount of exposed solute surface area affects how quickly the solution will dissolve. If coarse salt is used, less surface area is exposed, and the same amount of salt will dissolve more slowly. The salt diffuses through the water more quickly when the salt is finer because it exposes many more ions to the water. The salt eventually becomes uniformly dispersed throughout the glass and is no longer visible at the bottom.
Sugar in Water
Sugar undergoes a similar mechanism; however, sugar molecules differ from salt molecules. The sugar molecules are slightly polar rather than ionic compounds. Numerous OH groups in the sugar molecule result in natural dipoles. The solute molecule is broken apart as a result of interactions between these positive and negative regions and the positive and negative areas of the water molecules. Sugar may be dispersed uniformly throughout a cell in the same way that salt is in a solution. This is crucial for a variety of biological processes, including the synthesis of bigger molecules and energy. In other cases, cells have to actively move certain molecules out of the cytosol to keep the pH balance in check.
The presence of oxygen in seawater
Oxygen is an illustration of a gaseous solute. Oxygen is a polar molecule and is present as O2. As a result, the oxygen is naturally drawn to the polar water molecules. Oxygen is dissolved into the water as a result of air being mixed into the ocean by the waves and surface interactions with the atmosphere. Diffusion transports oxygen down the water column, supplying it to marine life all throughout the ocean. The oxygen in the ocean can sometimes be used by marine life more quickly than it can permeate into the water.
The presence of protons in the cytosol
To sustain correct cell activities, organisms of all types must control the number of solutes in their cells. The quantity of hydrogen ions (H+), or protons, present in the cytosol solution affects how acidic cells are in part. Because they are electronegative, the protons are drawn to the oxygen atoms in water. Cells use protons as a solute to perform critical tasks. Osmosis allows water to pass through cellular membranes, while hydrogen atoms are unable to do so. A potential force that may be employed to move other substances is produced by the concentration gradient.
References
- Kosower, E.M. (1969) “An introduction to Physical Organic Chemistry” Wiley: New York, p. 293
- Lew, Kristi (2009). “Homogeneous.” Acids and Bases, Essential Chemistry. New York: Chelsea House Publishing. Online publisher: Science Online. Facts On File, Inc. ISBN 978-0-7910-9783-0.
- Brown, T. L. (2009). Chemistry: The Central Science. Pearson Education.
