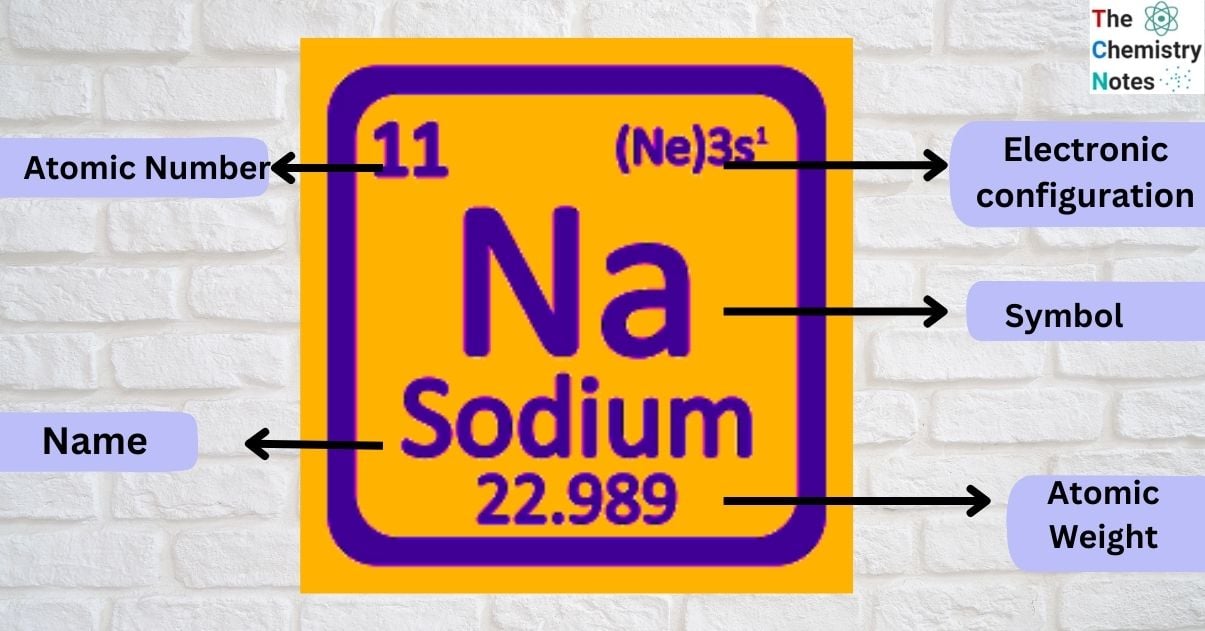
Sodium is a whitish metal that is easily melted and burnt. Sodium, which gets its name from the English word for “soda,” includes compounds that are quite useful in everyday life. Sodium is found in many common household items, like table salt (sodium chloride), baking soda (sodium bicarbonate), and a laundry detergent booster called borax (disodium tetraborate). Sodium is an alkali metal, with atomic number 11 in Group 1 [Ia] of the periodic table. Sodium (from the Latin natrium) is represented in chemical notation by the symbol Na.
History of Sodium
Sodium (Na) was isolated in a lab by Sir Humphry Davy.
- In 1807, Sir Humphry Davy electrolyzed molten caustic soda to extract this element.
- Sodium was difficult to extract from salt in the 1800s because it is reactive and quickly combined with other elements, despite the widespread usage of salt at the time.
- Davy, characterize it as a metal. As pure sodium may catch fire if exposed to moisture, he had to store it somewhere dry.
- Sodium is named after the English word “soda,” which was the original source of the element’s etymology. It’s also connected to the Medieval Latin word for a headache treatment, sodanum.
- Na in the periodic table represents sodium carbonate, which is derived from the Latin word natrium. One of the reasons sodium is represented by the letter Na is because of natron. Natron, a mineral salt mined from dry lake beds, was widely used as a preservative in ancient Egypt.
Occurrence of Sodium
- Sodium makes up 2.27 percent of the Earth’s crust and ranks seventh overall, after aluminum, iron, calcium, and magnesium but ahead of potassium as the most prevalent metal on the planet.
- It is believed that there are 10.8 grams of sodium per liter in the ocean. Sodium makes up around 1.05 percent of the ocean’s total composition, with a comparable halide concentration of about 3 percent It is never discovered in its elemental form because of its extreme reactivity.
- It occurs in a wide variety of insoluble and soluble minerals, including halite, natron, amphibole, and zeolite.
- Several types of silicates, including feldspars and micas, contain significant amounts of sodium.
- There are large rock salt deposits in several countries, and there are sodium nitrate deposits in both Chile and Peru.
- The spectra of stars, including Sun, and the interstellar medium have shown the presence of sodium in both its atomic and ionic forms.
- The silicate material found in meteorites contains, on average, around 4.6 sodium atoms for every 100 silicon atoms.
- Cryolite and feldspar, both sodium minerals, are insoluble because of the polymeric anions they contain; in the case of feldspar, these anions are polysilicates.
Isotopes of Sodium
The element sodium has thirteen different stable isotopes. The sole stable isotope of sodium is 23Na.
It has a fixed atomic mass of 22.98976928 atomic mass unit (u), making it a monoisotopic element. Both 22Na (half-life = 2.605 years) and 24Na (half-life 15 hours) are radioactive cosmogenic isotopes of sodium.
The longer-lived of the two nuclear isomers identified is 24mNa, having a half-life of about 20.2 ms. The neutron radiation dosage of a victim can be determined by measuring the concentration of 24Na relative to 23Na after exposure to acute neutron radiation, such as that which might result from a nuclear criticality event.
Elemental Properties of Sodium
| Electronic Configuration | [Ne] 3s1 |
| Atomic Number | 11 |
| Atomic Weight | 22.98977 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 1, 3, s-block |
| Density | 0.97 g.cm -3 at 20 °C |
| Covalent radius | 166±9 pm |
| Van der Waals radius | 0.196 nm |
| Electron shells | 2, 8, 1 |
| Electrons | 11 |
| Protons | 11 |
| Neutrons in most abundant isotope | 12 |
Physical Properties of Sodium
- Sodium is a soft, silvery metal that, when exposed to oxygen at room temperature and pressure, forms sodium oxide. This is why sodium is always stored in oil or inert gas: to prevent it from reacting.
- Due to just having one electron in its outermost shell, the resulting bonding is weak and the electron is free. As a result, it has high thermal and electrical conductivity.
- Sodium is so malleable that a knife may easily slice through it under normal conditions.
- Sodium and its compounds produce a yellow flame in a flame test because the excited 3s electrons release a photon as they decay from 3p to 3s. This photon has a wavelength of 589.6 nm, which is the same as the D line.
| Melting Point | 97.794°C, 208.029°F, 370.944 K |
| Boiling Point | 882.940°C, 1621.292°F, 1156.090 K |
| Density | 0.97 g cm-1 |
| Ionization Energies | 1st: 495.7 kJ.mol -1 |
| Heat of Vaporization | 97.42 kJ/mol |
| Heat of Fusion | 2.60 kJ/mol |
| Molar Heat Capacity | 28.230 J/(mol·K) |
| Standard Electrode Potential | – 2.71 V |
Chemical Properties of Sodium
- In its natural state, sodium is a very reactive metal. It forms sodium oxide when exposed to oxygen, which is a much less reactive compound. If it has any contaminants, its interaction with oxygen might speed up. A layer of sodium hydroxide is formed when sodium combines with water vapor or moisture; this layer then absorbs carbon dioxide to generate sodium bicarbonate. Sodium does not react with nitrogen. Because of this, sodium is typically stored in nitrogen or other inert gases or liquids. Liquid sodium is more reactive than solid sodium when exposed to air. Around 125 degrees Celsius, sodium liquid can catch fire. When burned in a dry environment, sodium produces a cloud of white smoke.
- When sodium is exposed to oxygen in the absence of moisture, sodium monoxide (Na2O) is often produced. In an autoclave (a heated pressure vessel) with oxygen at high pressure, metallic sodium may be heated to 300 °C (570 °F) to produce the superoxide (NaO2). Sodium peroxide, Na2O, with a high surface area, can also be oxidized to produce superoxide.
- Alkali metals are frequently engaged in reactions involving halogens. The reactivity of a halogen grows or shrinks as its atomic weight grows or shrinks. Sodium fits that mold, too. There is chemiluminescence when halogens and sodium vapours react. Sodium and halogen acids, such as hydrochloric acid, react violently to generate sodium halides. This reaction produces a lot of heat.
- Salts are formed when sodium interacts with strong metal acids. Sodium nitrate is formed when sodium reacts with nitric acid vapors at 15 °C. Sodium becomes sodium sulfate and sodium acetate when it reacts with sulfuric and acetic acids, respectively. It forms polysulfides when exposed to molten sulfur. Strong reactions between liquid tellurium and solid sodium result in tellurides and selenides, respectively.
- The interactions of sodium with organic molecules have been extensively explored. In the presence of anhydrous alcohol, sodium forms alkoxides.
- Methanol is the most reactive alcohol, whereas higher molecular weight alcohols are progressively less reactive. Sodium methoxide is formed when sodium reacts with an excess of methanol. Sodium is converted into sodium salts by reactions with organic acids.
- At temperatures over 100 °C (212 °F), pure sodium absorbs hydrogen at an increasing rate. At temperatures exceeding 350 °C (660 °F), pure sodium hydride may be created by subjecting sodium to a high flow rate of hydrogen gas. Compared to lithium hydride and potassium hydride, sodium hydride has a much higher dissociation temperature at which it produces hydrogen and molten sodium.
- Despite the low degree of reactivity between sodium and carbon, lamellar (layerlike) materials can be created using sodium between graphite layers. Carbon monoxide combines with sodium to produce sodium carbide and sodium carbonate at a temperature of 625 °C (1,157 °F).
- All transition metal oxides can be reduced to their elemental forms by treating them with sodium, with the exception of those containing Group 4 (IVb) elements (titanium, zirconium, and hafnium). Many metallic halides react with sodium, with the metal being displaced from the salt and a sodium halide being formed. Titanium and tantalum, for example, are both transition metals that are produced using this mechanism.
- Liquid ammonia is frequently employed as a sodium solvent, allowing a variety of processes that would otherwise require heat to take place at room temperature. For example, sodium superoxide (NaO2) may be produced by passing oxygen through sodium ammonia solutions at -77 °C (-107 °F). Ammonia is also used as a solvent in sodium reactions with arsenic, tellurium, antimony, bismuth, and a variety of other low-melting metals. To prepare the surface of polytetrafluoroethylene (Teflon) for cementing to other materials, sodium-ammonia solutions are employed to blacken it. Because of their strong reducing power, sodium-ammonia solutions are effective in a variety of chemical processes known as Birch reductions.
- All of the alkali metals dissolve in liquid ammonia to generate bright blue solutions, and at normal temperatures, a gradual reaction between sodium and ammonia happens to form sodamide, NaNH2, and hydrogen, analogous to the interaction of sodium with water to form NaOH and hydrogen.
The reactions are as follows:
Na + NH3 → NaNH2+ 1/2 H2
Na + H2O → NaOH + 1/2 H2
Several metals and metal oxides can catalyze the reaction of alkali metal-ammonia solutions to create amide and hydrogen.
Uses/Applications of Sodium
- The most common form of sodium chloride is table salt, which is utilized in a number of applications. It is an incredibly versatile flavoring component that is utilized in practically every sort of meal imaginable. It is also advantageous in that it makes the curing process more practicable. Cured items, such as meats, are preserved by using a high concentration of salt chloride. Moreover, the element may be found in baking soda, a popular household item.

- Baking soda is primarily used in the making of cakes and other baked products as a leavening agent. Other than increasing the flavor of food, sodium bicarbonate and sodium chloride perform a number of roles. Both have a wide range of applications that aren’t limited to the kitchen. When baking soda and vinegar are mixed, they create a powerful cleaning agent that can be used on almost any surface. Another ubiquitous chemical with which we come into touch on a daily basis is sodium fluoride.
- Sodium fluoride is also utilized in water treatment systems to treat water. It is used in a variety of industries, including wood preservation, glass frosting, and stainless steel prickling. It is also used as a preservative in a range of adhesive and glue compositions. Sodium is also employed in a wide range of medical operations. The most often used application is rehydration treatment, which is the most widely used application. This technique may aid those who are dehydrated as a consequence of diarrhoea or other health issues.
- A solution comprising potassium chloride, sodium chloride, and glucose can help replace our bodies’ water levels. A very simple but crucial answer has been identified, which has saved a considerable number of lives. Beautiful lighting fixtures, such as chandeliers, are also made from sodium. Because of their inexpensive cost, sodium lamps are commonly utilized in street lighting. They are easily identified by their yellow-orange light, which distinguishes them almost anywhere on the world. In addition to lamps, sodium is utilized in the manufacture of a variety of other items. It is used in the manufacture of pottery, soap, glass, and textile colors, among other things.
- Sodium is employed in a variety of sectors. Sodium is utilized in the manufacturing of titanium metals. Metallic sodium is an important component in the manufacture of organic compounds. Sulfur dioxide can be used to enhance the structure of alloys. In addition to other applications, sodium is utilized in the separation of potassium and zirconium from their respective compounds. It is utilized in chemical heat transfer and is a vital component in the creation of artificial rubber, which has several uses in a wide range of industries.
- It is also used to improve the structure of some alloys, soaps, molten metal purification, and sodium vapour lamps.
- Sodium is a component of sodium chloride, a critical molecule in the living environment.
- Sodium is essential in the production of organic molecules and esters.
- Making glass necessitates the use of solid sodium carbonate.
Chemical Reactions of Sodium
Reaction of Sodium and Water
When sodium reacts with cold water, it produces hydrogen and a colorless solution of sodium hydroxide.
2Na + 2H2O → 2NaOH + H2
Reaction of Sodium and Oxygen
When sodium is exposed to oxygen, it produces a combination of sodium oxide and sodium peroxide, which burns with an orange flame and cools to a white solid.
For the simple oxide:
4Na + O2 → 2Na2O
For the peroxide:
2Na + O2 → Na2O2
Reaction of Sodium and Alcohol
Sodium reacts with anhydrous alcohols to make the corresponding alcoholates (or alkoxides), according to
Na + ROH → RONa + 1/2 H2
where;
R represents the organic part of the alcohol (R = CH3 for methanol, CH3CH2 for ethanol, and so on).
The reaction is vigorous with methanol and slows as the molecular weight of the alcohol increases. On a large scale, sodium methoxide is created by reacting sodium with excess methanol. Sodium salts are formed when organic acids react with sodium.
Reaction of Sodium with Haloalkanes
The significant negative free energy of sodium halide creation allows for the dehalogenation of a variety of organic halides, with sodium halide formation being energetically preferred. The so-called Wurtz reaction, which is based on this concept, is widely employed in organic synthesis:
2RCl + 2Na → R―R + 2NaCl
Octane may be synthesized from bromobutane and sodium using this method. A variety of organosodium compounds have the sodium atom directly attached to a carbon atom, such as methylsodium, NaCH3. Such compounds can be synthesized by reacting sodium with mercury dialkyls or diaryls, as shown in the following equation:
Hg(CH3)2 + 2Na → 2NaCH3 + Hg
A variety of halogenated hydrocarbons react aggressively with sodium. When a combination of carbon tetrachloride and sodium is shocked, for example, a powerful explosion ensues. Even when the salt is greatly diluted, as in sodium amalgam, a rapid reaction with carbon tetrachloride occurs.
Health Effects of Sodium
Numerous foods include sodium in the form of salt or other sodium-containing compounds. Humans require a stable fluid balance for optimal physical functioning. Sodium is also necessary for proper muscle and nerve function. Too much salt is associated with an increased risk of hypertension and renal damage.
Some people get as little as 2 g/day, while others get as much as 20 g/day of sodium, although this varies greatly from person to person and culture to culture. The amount of sodium in the diet is controversial despite the fact that it is important.
Sodium hydroxide vapors, which are produced when sodium comes into contact with water, particularly sweat, are extremely irritating to the skin, eyes, nose, and throat. Sneezing and coughing might be side effects. Extreme exposure might cause bronchitis and other respiratory problems. Itching, tingling, heat and caustic burns, and perhaps permanent damage, may result from skin contact. If this comes into contact with your eyes, you might perhaps go blind.
The kidneys often struggle to remove excess salt from the blood. The body stores water in response to an increase in salt levels. This results in a rise in intravascular fluid pressure and total blood volume. The heart has to work harder, and blood vessel pressure rises, when there is more blood in circulation. Long-term exposure to the added stress can cause blood vessels to harden, which in turn increases the risk of hypertension, heart attack, and stroke. In addition, it can cause cardiac failure. Too much salt may be unhealthy for your bones and may damage your heart and aorta without raising your blood pressure.
Deficiency of Sodium
Abnormally low sodium levels in the blood are medically referred to as hyponatremia.
Sodium is added to many processed meals and exists naturally in other foods, therefore sodium shortage is uncommon in the United States. Hyponatremia is common in the elderly, especially those who are institutionalized or use drugs that cause the body to lose sodium (such as those in long-term care institutions or hospitals). Hyponatremia can be brought on by excessive perspiration, diarrhea, or vomiting if salt is lost in those fluids. Diseases including heart failure and liver cirrhosis can cause inappropriate accumulation of fluid, leading to hyponatremia. In extremely unusual circumstances, hyponatremia can be caused by merely consuming too much fluid if the kidneys are unable to eliminate the excess water. Nausea, vomiting, headaches, mental confusion, drowsiness, seizures, and coma are all possible outcomes of hyponatremia.
Excess of Sodium
Hypernatremia is the medical term for high blood salt levels.
Elderly people with cognitive and physical impairments may get acute dehydration if they don’t consume enough fluids or food, or if they’re ill with a high fever, vomiting, or illness. Other factors include diuretic drugs and excessive perspiration. When blood sodium levels rise, cells release water into the blood to dilute the salt. Seizures, unconsciousness, and even death can result from an accumulation of fluid in the brain brought on by this fluid shift. Difficulty breathing is a common symptom of lung fluid accumulation. Nausea, vomiting, weakness, lack of appetite, excessive thirst, disorientation, and renal impairment are some of the other symptoms of hypernatremia.
Environmental Effects of Sodium
- The powdered form of sodium is highly explosive in water and poisonous in many different configurations.
- The median tolerance level (TLM) for mosquito fish in fresh water is 125 ppm/96 hours, while the TLM for bluegill in municipal water is 88 mg/48 hours.
- Fate in the environment: this chemical is immobile in solid form but readily absorbs moisture. Once sodium hydroxide becomes liquid, it can quickly permeate the soil and potentially contaminate groundwater.
Have a look and watch the video
References
- Isotope masses from Ame2003 Atomic Mass Evaluation by G. Audi, A.H. Wapstra, C. Thibault, J. Blachot and O. Bersillon in Nuclear Physics A729 (2003).
- Smith, Michael (12 July 2011). Organic Synthesis (3 ed.). Academic Press, 2011. p. 455. ISBN 978-0-12-415884-9.
- Solomons; Fryhle (2006). Organic Chemistry (8 ed.). John Wiley & Sons, 2006. p. 272. ISBN 978-81-265-1050-4.
- https://unacademy.com/content/neet-ug/study-material/chemistry/the-uses-of-sodium/
- National Academies of Sciences, Engineering, and Medicine 2019. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press. https://doi.org/10.17226/25353.
- He J, Gu D, Chen J, Wu X, Kelly TN, Huang JF, Chen JC, Chen CS, Bazzano LA, Reynolds K, Whelton PK. Premature deaths attributable to blood pressure in China: a prospective cohort study. The Lancet. 2009 Nov 21;374(9703):1765-72.
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013 Apr 4;346:f1326.

