Silicon (Si) is a non-metallic chemical element in the carbon family (Group 14 [IVa] of the periodic table). Silicon is the most prevalent electropositive element in the Earth’s crust. It is a metalloid that has a distinct metallic sheen and is exceedingly fragile. Earth’s crust contains 27.7% silicon. The word silicon is derived from the Latin silex or silicis, which means “flint” or “hard stone.”
Silicon is the eighth most abundant element in the universe by mass, yet it is only extremely rarely found as a pure element in the Earth’s crust. It exists in a vast variety of forms as silicon dioxide (silica), silicates, planets, and cosmic dusts across space. Silicate minerals make up more than 90% of the Earth’s crust, making silicon (approximately 28% by mass) the second most prevalent element in the crust after oxygen.
History of Silicon
Natural silicon-based materials have been utilized for thousands of years due to the availability of silicon in the Earth’s crust. Several ancient cultures were aware with silicon rock crystals, including the predynastic Egyptians and the ancient Chinese, who utilized it to make beads and miniature vases. Since at least 1500 BC, the Egyptians and the ancient Phoenicians have produced glass that contains silica. Natural silicate compounds were also utilized in various types of mortar for the construction of early human houses.
Antoine Lavoisier hypothesized that silica may be an oxide of a fundamental chemical element in 1787, but he lacked the tools necessary to decrease the oxide and isolate the element due to silicon’s strong chemical affinity for oxygen.
Gay-Lussac and Thénard are considered to have created impure amorphous silicon in 1811. Through the heating of freshly isolated potassium metal with silicon tetrafluoride.
Scottish scientist Thomas Thomson gave silicon its current name in 1817.
Jöns Jakob Berzelius initially prepared it and gave a pure form’s description of it in 1823.
Jöns Jacob Berzelius created amorphous silicon in 1824 through the reduction of potassium fluorosilicate with molten potassium metal, a process that is similar to that performed by Gay-Lussac, however he further purified the end result into a dark powder by putting it through several washings. He is typically awarded credit for discovering the element.
Occurrence of Silicon
Silicon is never found in its native state, but rather in silica-rich rocks such as obsidian, granite, diorite, and sandstone as the silicate ion SiO44- in conjunction with oxygen. Two of the most important silicate minerals are feldspar and quartz.
After oxygen, Si is the second most common element in the crust of the Earth, and it is the eighth most common element overall in the universe. The form of silicon dioxide (silica) is the most typical. Nearly 75 percent of the crust of our planet is made up of the two elements silicon and oxygen.
The most prevalent substance in the crust of the planet is silicon dioxide (SiO2), the most typical component of silicon. Ordinary sand is one of its most prevalent forms, although it may also be found as quartz, rock crystal, amethyst, agate, flint, jasper, and opal. A lot of silicon dioxide is utilized in the production of bricks and glass. Silica gel, a colloidal form of silicon dioxide, is employed as a desiccant because it readily absorbs moisture.
- Pure Si is too reactive to be found in nature, but it is present in almost all rocks, as well as sand, clay, and soils, either alone or in silicates with other elements like oxygen and aluminum, magnesium, calcium, sodium, potassium, or iron.
- The oxidized form, in the form of silicon dioxide and in particular silicates, is also prevalent in the Earth’s crust and plays a significant role in the composition of the mantle.
- All natural waterways, the environment (as siliceous dust), numerous plants, and the bones, tissues, and bodily fluids of certain animals also include its components.
Isotopes of Silicon
Three stable isotopes (²⁸Si, ²⁹Si, and ³⁰Si) of silicon exist in nature. Silicon isotopes are employed in industrial and scientific applications.
| Isotope | Natural abundance (atomic %) |
|---|---|
| ²⁸Si | 92.22 |
| ²⁹Si | 4.69 |
| ³⁰Si | 3.09 |
Si-28 is being studied in order to increase semiconductor thermal conductivity.
Si-29 is widely used in NMR spectroscopy.
Si-30 was used to create the radioisotope Si-31. Si-30 has also been used to investigate Silicon’s self-diffusivity and the isotope influence on superconductivity.
Allotropes of Silicon
Silicon has several allotropes, but the two most prevalent are amorphous silicon and crystalline silicon.
Amorphous Silicon
Amorphous silicon is a dark powder. The atoms in the structure are mainly linked throughout, however, some atoms are not fully bound. As a result, amorphous silicon is flexible but not very robust. Because silicon is a semiconductor (a substance that can serve as both a conductor and an insulator), amorphous silicon is an excellent material for solar panels. Solar cells, in particular amorphous silicon, are utilized in calculators, watches, and other devices.
Crystalline Silicon
Crystalline silicon (also known as polycrystalline silicon) is a metallic grey solid. It has the appearance of tiny crystals. Crystalline silicon is stronger and more stable than amorphous silicon because the atoms in the structure are entirely linked throughout. Crystalline silicon, like amorphous silicon, is used in solar cells. It’s also found in microchips in electronics, such as the computer or smartphone you’re using to read this lesson.
Elemental Properties of Silicon
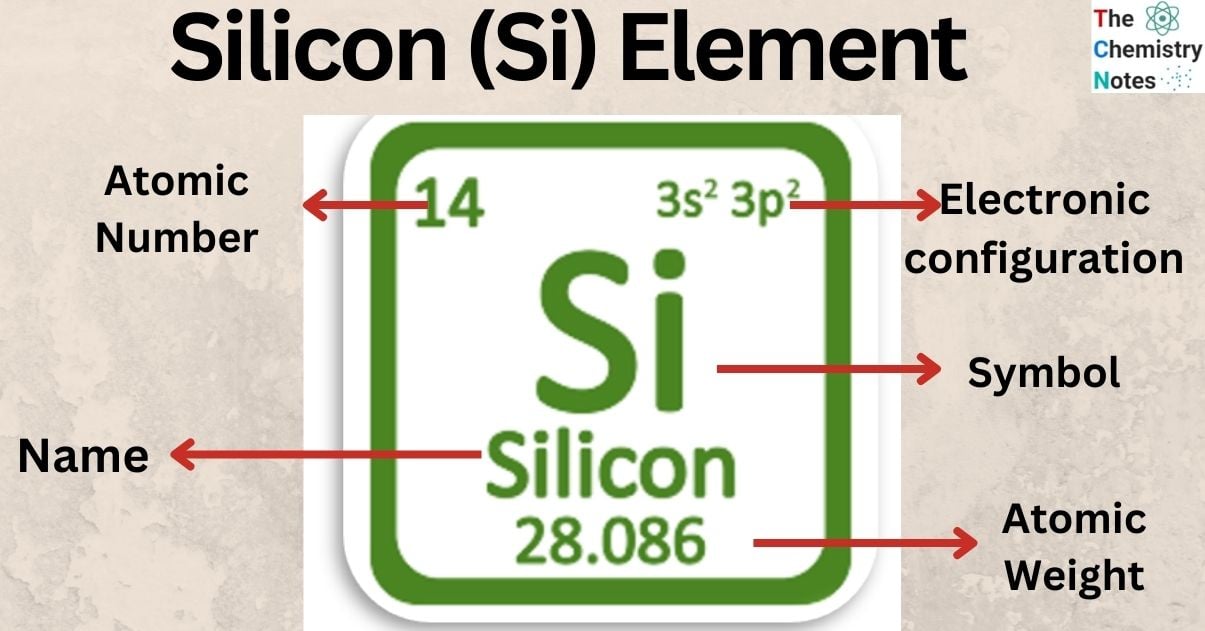
| Electronic Configuration | [Ne] 3s2 3p2 |
| Atomic Number | 14 |
| Atomic Weight | 28.085 |
| State | solid at 298 K |
| Group, Period, and Block | 14, 3, p-block |
| Density | 2.33 g.cm -3 at 20 °C |
| Covalent radius | 111(2) pm |
| Van der Waals radius | 210 pm |
| Electron shells | 2, 8, 4 |
| Electrons | 14 |
| Protons | 14 |
| Neutrons in most abundant isotope | 14 |
Physical Properties of Silicon
- Chemical element silicon has the symbol Si and atomic number 14.
- It is a crystalline solid that has a blue-gray metallic luster and is hard and brittle.
- Si is the tenth most abundant element in the universe by mass, yet it is extremely rare in nature as a pure element. It is commonly found as silicon dioxide or silicates.
- It is both a semiconductor and a tetravalent metalloid. Since Si is a semiconductor, which means that it has a restricted capacity to conduct electricity. The capacity of silicon to conduct electricity may be significantly improved by doping it with additional elements. Si is used to make semiconductor devices such as transistors, microchips, and solar cells.
| Color/physical appearance | Pure silicon is a hard, dark gray solid |
| Melting point/freezing point | 1414°C, 2577°F, 1687 K |
| Boiling point | 3265°C, 5909°F, 3538 K |
| Density | 2.3296 g cm−3 |
| Malleability | No |
| Ductility | No |
| Electronegativity | 1.9 (Pauling Scale) 1.916 (Allen Scale) |
| Phase | Solid |
| Luster | Yes, A metallic shine or glow |
| Allotropes | Silicon has two allotropic forms, a brown amorphous form, and a dark crystalline form |
Chemical Properties of Silicon
At room temperature, silicon is a rather passive element. It does not react with oxygen or the majority of other elements. Water, steam, and the majority of acids have minimal effect on the element. However, at higher temperatures, Si becomes considerably more reactive. It mixes with oxygen, nitrogen, sulfur, phosphorus, and other elements when molten (melted). It also easily produces a variety of alloys when melted.
- Si forms compounds with metals (silicides) and with non-metals.
- Combined with oxygen as silica (silicon dioxide, SiO2) or with oxygen and metals as silicate minerals. It is stable in air even at elevated temperatures owing to the formation of a protective oxide film.
- It has dark-brown crystals that burn in air when ignited.
- It is transparent to long-wavelength infrared radiation.
- It dissolves only in a mixture of nitric acid and hydrofluoric acid
Chemical Reaction of Silicon
Reaction of Silicon with air:
Under normal conditions, silicon is passivated by a thin layer of SiO2 on the surface and does not react with air.
- When heated over 900°C, Si interacts with oxygen, O2, to generate SiO2:
Si (s) + O2 (g) → SiO2 (s)
- Si interacts with nitrogen, N2, when heated beyond 1400 °C, producing the silicon nitrides SiN and Si3N4:
2 Si (s) + N 2(g) → 2 SiN (s)
3 Si (s) + 2 N2 (g) → Si3N4 (s)
Reaction of Silicon with acids:
Under normal circumstances, silicon does not react with most acids. It is dissolved by hydrofluoric acid, HF, as the driving force, most likely due to the complex production of [SiF6]2-.
Si (s) + 6 HF (aq) ⇌ [SiF6]2− (aq) + 2H+ (aq) + 2 H2 (g)
Reaction of Silicon with bases:
When Si combines with hot alkali solutions, silicate ions, SiO32-, are formed :
Si (s) + 2 OH−(aq) → SiO32− (aq) + 2 H2 (g)
Reaction of silicon with water
Si does not react with water, even as steam, under normal conditions.
Reaction of silicon with halogens
Si reacts halogens, forming the corresponding Si(IV) halides.
Si (s) + 2 F2 (g) → SiF4 (g)
Si (s) + 2 Cl2 (g) → SiCl4 (l)
Si (s) + 2 Br2 (g) → SiBr4 (l)
Si (s) + 2 I2 (g) → SiI4 (s)
Uses of Silicon
More than 90% of the earth’s crust is made up of silicon silicate minerals. These natural silicon compounds are employed in industry and do not need extensive processing or purification.
Furthermore, Si is employed in advanced applications where purity is critical. Let’s take a closer look at the numerous applications of Si. Si has applications in an array of sectors for a multitude of reasons. The major sectors that require silicon are identified below.
Electronic Uses
Silicon is highly valued in the electronics industry. Pure Si has a high electrical resistance and a poor electrical conductivity.
- Si is a semi-conductor, which means that it conducts electricity under certain circumstances while acting as an insulator under others. Because of these properties, it is an excellent material for manufacturing transistors that amplify electrical impulses.
- Si is used to coat electrical components such as keypads, keyboards, and copier rollers, as well as hard coatings for computers, fax machines, telephones, and home entertainment devices.
Construction Industry
- Different types of construction materials such as portland cement, fire bricks, are made of silicon.
- Various waterproofing materials used for the constructions now days are made of silicons.
- Earth bricks contains 50-60 percent of silica which prevents cracking and shrinking of the bricks.
- Silicon waxes and greases are employed in high-temperature environments.
- Si compounds are also utilized to make mechanical seals, molding compounds, and waterproofing materials.
Ceramics
Silica is a component ingredient of numerous ceramics, including whiteware ceramics containing burned clay minerals.
- The main component of porcelain is kaolinite, a silicate mineral. The classic soda-lime glass used in window panes and containers also contains Si compounds.
- Optical fibers are silica-based glass fibers of various types. Glass wool, used for thermal insulation, and fiberglass, used for structural support, are both silica-based compounds.
- Silicon carbide is very useful in the production of high-strength ceramics.
Industrial Uses
- Si aids in the insulation of structures against the weather. It contributes to increased energy efficiency and the maintenance of acceptable temperatures for both humans and machines.
- In addition, the application of silicon as a coating aids in the structural integrity and resistance to corrosion of buildings, roads, and bridges.
- Silumin, a silicon-aluminum alloy, is used to cast components in the automotive sector. This combination solidifies with little thermal contraction, enhancing aluminum’s stress and wear resistance qualities.This decreases the likelihood of rips and cracks on the vehicle’s surface.
- Ferrosilicon is an alloy of silicon and cast iron. When exposed to air, it inhibits the production of cementite. Due to Si high oxygen efficiency, ferrosilicon is particularly useful in the steel sector for preventing corrosion.
Biological Importance of Silicon
Silicon is also required for the survival of plants and vegetables. Phytolites are microscopic silica particles generated by some plants. These particles can also be preserved in fossils, giving evolutionary data. It is an essential mineral in our bodies.
- Silicon is found in the cells that produce cartilage and bone, in the collagen that resides in the skin and is responsible for its flexibility, and in the connective tissue that protects the body’s structures.
- Silicon aids in the creation of collagen, the primary component of connective tissue.
- It strengthens and makes blood vessel walls more flexible, reduces capillary permeability, speeds up healing processes, has sebostatic action, and strengthens hair and nails.
- This element promotes the phosphorylation of proteins, saccharides, and nucleotides.
- It is also required for the creation of the cytoskeleton and other mechanical or supporting cellular structures.
- Silicon is the first substrate used to create silicones. These are synthetic polymers with silicon atoms linked together by oxygen bridges.
- Because of their most convenient physical and chemical properties: moistening and film-forming, giving liquid shape, and boosting solubility, they are utilized in practically all types of goods.
- Silicon acids, like active carbon, produce colloid gel, silica gel, which has absorptive properties.
- Pharmaceuticals, sealants, caulks, adhesives, paints, herbicides, fungicides, and computer and television screens are all examples of organo-silicon compounds.
Health Effects of Silicon
As silicon makes 27.7 percent of earth’s crust by mass and second most abundant element after oxygen. It is hard to avoid the silicon as a human being. Due to the application of Si in different sectors such as construction, electronics human are encountering silicons on everyday basis.
- Lung Disease
Crystalline silica (silicon dioxide) is a dangerous respiratory irritant. However, the possibility of crystalline silica formation during normal processing is quite rare. The oral LD50 is 3160 mg/kg. With such abundance comes some challenges to human bodies. While mining the silicon miners may breath silica containing dust which may lead to silicosis. Articles of silica dust become stuck in lung tissue, causing inflammation and scarring.
The particles also decrease the ability of the lungs to absorb oxygen. This is referred to as silicosis. Silicosis is a gradual, debilitating, and occasionally deadly condition that causes chronic lung damage.
Occupational exposure to crystalline silica, notably quartz and cristobalite, has been linked to lung cancer. Studies on miners, diatomaceous earth workers, granite workers, pottery workers, refractory brick workers, and other occupations have found an exposure-response association.
- Skin Inflammation
On touch, silicon crystalline irritates the skin and eyes. Lung and mucous membrane inflammation will result from inhalation. Watering and redness are symptoms of ocular irritation. Skin inflammation is characterized by reddening, scaling, and itching.
- Other effects
- Several epidemiological studies have found statistically significant increases in the frequency of fatalities or occurrences of immunologic disorders and autoimmune illnesses among silica-exposed employees.
- Scleroderma, rheumatoid arthritis, systemic lupus erythematosus, and sarcoidosis are examples of these illnesses and disorders.
- Recent epidemiological investigations have found statistically significant links between occupational crystalline silica exposure and renal illnesses and subclinical renal alterations.
- Crystalline silica can weaken the immune system, resulting to mycobacterial (tuberculous and nontuberculous) or fungal infections, particularly in silicosis workers.
- Bronchitis, chronic obstructive pulmonary disease (COPD), and emphysema have all been linked to occupational exposure to breathing crystalline silica.
- According to certain epidemiologic research, these health impacts may be less common or nonexistent among nonsmokers.
Environmental Effects of Silicon
Although there are some researches been conducted to determine whether silicon has any adverse effect on environment. However ,some small effects are pointed out by some researchers but there is no such concrete evidence that has surfaced to back the study till date. May be future studies could make clear statements on effects of Si on our environment.
Fun and Important Facts of Silicon
- Silicon and silicone are not similar at all, despite what some people may believe. Silicon is a naturally occurring element that appears as element 14 on the periodic table. Silicone is a synthetic substance composed of silicon-oxygen polymers that is utilized in a wide range of applications.
- The Apollo 11 astronauts left behind a white bag holding a silicon disc somewhat larger than a silver dollar when they landed on the moon in 1969.
- According to the Institute of Materials, Minerals, and Mining, silicon carbide (SiC) is nearly as hard as diamond. It has a Mohs hardness of 9-9.5, which is somewhat less than diamond, which has a hardness of 10.
- The silicon used in computer chips gave rise to the term Silicon Valley. In 1971, the moniker first appeared in the publication “Electronic News.”
- The Avogadro Project aims to create a standard kilogram mass from a single silicon crystal. These spheres are said to be the roundest man-made things ever built.
- Commercially, silicon is purified by heating sand and carbon in an electric furnace. A molten salt electrolysis process is used to obtain ultrapure silicon.
- Silicones (silicon-oxygen-hydrocarbon compounds) are a class of materials that vary from liquids to hard solids and have a wide range of applications, including usage as adhesives, sealants, lubricants, and insulators.
- Diatoms take silicon from the environment and integrate it into their cell walls.
- Aerolites are silicon-containing meteorites.
Si is the second most abundant element, it even exists in our own bodies. What do you know about it? Watch out this interesting video.
References
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. ISBN 978-0-19-927029-3.
- https://www.rsc.org/periodic-table/element/14/silicon
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- https://www.lenntech.com/periodic/elements/si.html
- Mary Elvira Weeks, Discovery of the Elements., (2003) p162, Kessinger Publishing Reprints.
- An overview of silica in biology: its chemistry and recent technological advances: https://doi.org/10.1007/978-3-540-88552-8_13
- John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements., (2002) p387. Oxford University Press.
- H. R. Huff, U. Gösele, H. Tsuya, Semiconductor Silicon., (1998) p70, The Electrochemical Society.
- King, R. Bruce (1995). Inorganic Chemistry of Main Group Elements. Wiley-VCH. ISBN 978-0-471-18602-1.
- https://www.webelements.com/silicon/chemistry.html#:~:text=Reaction%20of%20silicon%20with%20air,the%20air%20gives%20silicon%20dioxide.
- Zulehner, Werner; Neuer, Bernd; Rau, Gerhard. “Silicon”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_721
- https://www.marnys.com/en/magazine/art0201-the-importance-ionized-minerals-living-beings/
- [Biological function of some elements and their compounds. IV. Silicon, silicon acids, silicones]: PMID: 19999810

