The shapes of organic molecules are best visualized using molecular models. Organic compounds have three dimensions, and the preferred spatial arrangement of the covalent bonds determines how they are shaped.
The existence of double and triple bonds is explained by valence bond theory and molecular orbital theory, which include sigma and pi bonds. When the available orbitals with the highest energies of the individual atoms overlap, sigma bonds are created. As a result, the two atoms will form a sigma bond with a higher electron density sharing between the two nuclei as a result of the positive interaction.
Atomic orbitals, hybridization, and hybrid orbitals are all concepts that must be understood on a fundamental level in order to comprehend Sigma and Pi bonds and shapes of organic molecules.
Hybridization
In chemistry, hybridization is the idea of combining two atomic orbitals to create a new class of hybridized orbitals.
Typically, this mixing creates hybrid orbitals with completely different energies, shapes, etc. In hybridization, the same-energy level atomic orbitals are primarily involved. However, both fully filled and half-filled orbitals can participate in this process if their energies are equal.
Atoms use hybridization to achieve the lowest possible energy state.
The primary goal is to keep lone pairs and bonds as far apart as possible in the absence of additional complicating elements (like conjugation and aromaticity).
Hybridization Types
The types of orbitals involved in the mixing can be used to categorize the hybridization as sp3, sp2, sp, sp3d, sp3d2, or sp3d3.
sp Hybridization
When two s and two p orbitals within an atom’s main shell combine to form two new equivalent orbitals, this process is known as sp hybridization. The newly created orbitals are known as sp hybridized orbitals. With a 180° angle, it creates linear molecules.
This type of hybridization involves the mixing of one ‘s’ orbital and one ‘p’ orbital of equal energy to produce a new hybrid orbital known as a sp hybridized orbital.
sp2 Hybridization
When an atom’s s and p orbitals from the same shell combine to form three equivalent orbitals, this process is known as sp2 hybridization. Sp2 hybrid orbitals are the new orbitals that have formed.
Trigonal hybridization is another name for sp2 hybridization. Trigonal symmetry was used to form a mixture of s and p orbitals, which are maintained at 120°.
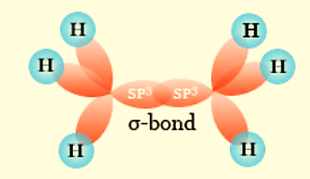
sp3 Hybridization
Tetrahedral hybridization, or sp3, is the process by which one’s’ orbital and three ‘p’ orbitals from the same atomic shell combine to form four new equivalent orbitals. Sp3 hybrid orbitals are the new orbitals that have formed.
These form an angle of 109°28′ with one another and are pointed at the four corners of a regular tetrahedron.
The sp3 hybrid orbitals are at an angle of 109.28° degrees.
Sigma bond
Sigma bonds (σ) are the first type of covalent bond formed by the head-to-head overlap between two atoms.
They are only present in single bonds, as well as double and triple bonds.
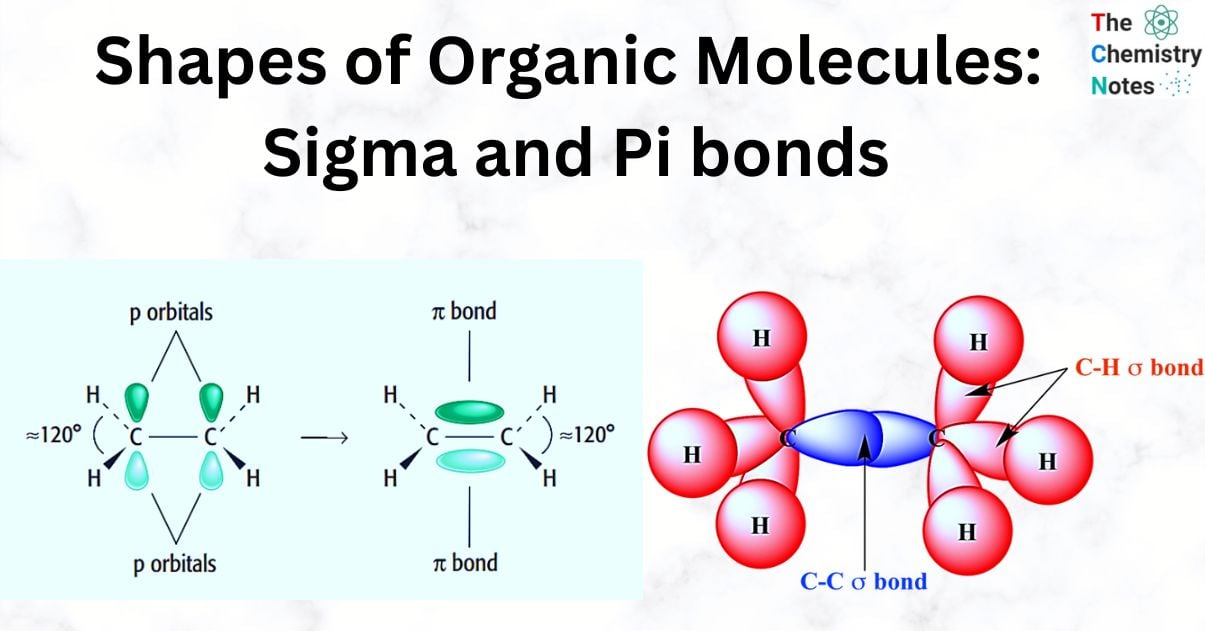
With an electronic configuration of 1s22s22p2, each carbon atom has six electrons. This indicates that the outermost shell of carbon has four electrons. A carbon atom can acquire the electronic configuration of the noble gas neon by forming solitary covalent bonds with four additional atoms. These single covalent bonds are sigma bonds (σ).
The two electrons that make up a bond are located in a lobe between the nuclei of the two atoms that are sharing them. The two positively charged nuclei and the negatively charged electrons interact electrostatically to form a bond that holds the atoms together.
Each carbon atom forms four bonds in the majority of organic compounds. Each carbon atom has four pairs of bonding electrons that are in opposition to one another.
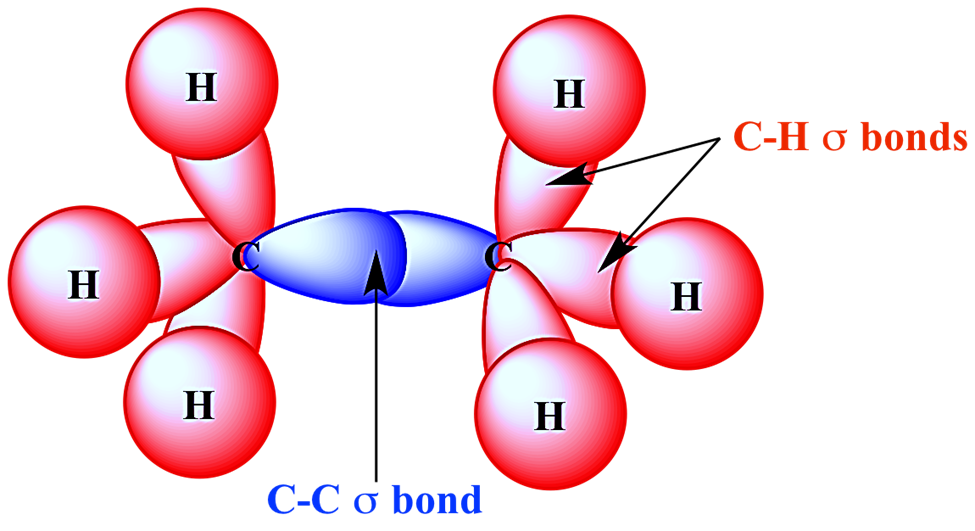
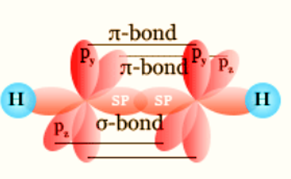
Pi Bond (π)
Pi bonds (π) are the second and third types of covalent bonds formed between two atoms by side to side p orbital overlap.
They can be found only in double and triple bonds.
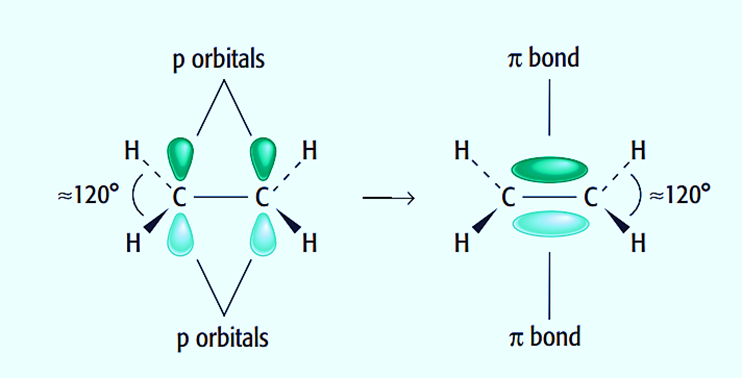
In organic molecules, carbon atoms can combine in double bonds in addition to single bonds. Alkenes like ethene contain C=C double bonds, which are composed of a (σ) bond and a pi (π) bond. The double bond’s carbon atoms will each form three bonds, which is an example of sp2 hybridization. This results in one extra outer electron remaining in a 2p orbital for each carbon atom. These two p orbitals come together to create a bond. In an ethene molecule, the two lobes that make up the bond are located above and below the plane of the atoms. The p orbitals’ overlap is maximized in this way.
Each of the two carbon atoms that make up the double bond is encircled by three pairs of electrons. These are all in the molecule’s plane and repel one another, resulting in bond angles of about 120 degrees.
Shapes of Organic Molecules
The overall shapes of organic molecules is determined by the shape of the central carbon atoms that make up the molecule’s backbone. The types of hybrid orbitals that make up the bonds between the central carbon atoms determine the backbone of the shapes of organic molecules. A tetrahedral molecule will exist if the central carbon atoms are sp3 hybridized. Sp2 hybridization of the central carbon atoms results in trigonal-planar shapes, whereas sp hybridization results in linear molecules.
Ethane
Ethylene

Acetylene
Find out more about Sigma and pi bonds in this video.
References
- https://courses.lumenlearning.com/suny-potsdam-organicchemistry/chapter/2-1-combining-atomic-orbitals-s-and-p-bonding/
- W. Locke (1997). Introduction to Molecular Orbital Theory. Retrieved May 18, 2005.
- https://www.savemyexams.co.uk/as/chemistry/cie/19/revision-notes/3-organic-chemistry/3-1-an-introduction-to-organic-chemistry/3-1-6-shapes-of-organic-molecules-sigma-and-pi-bonds/
- Carl R. Nave (2005). HyperPhysics. Retrieved May 18, 2005.
- https://www.futurelearn.com/info/courses/everyday-chemistry/0/steps/22294

