Separation of mixtures is applicable in order to either separate the unnecessary components from a mixture or separate the required components from a mixture. The physical blending of two unlike or related compounds in liquids, colloids, or suspensions produces a substance. These compounds identities are also preserved. This is referred to as a mixture. However, they do not react chemically and are not in a certain ratio. Specific physical characteristics are present in each of the constituent parts that make up the mixture.
Solutions can be divided into two categories: homogeneous mixtures and heterogeneous mixtures.
Types of Mixtures
There are two types of mixtures which are discussed here:
Homogeneous Mixture: A homogeneous mixture has the same component ratios throughout the sample, whether solid, liquid, or gaseous. The composition of it is constant throughout. In a homogenous mixture, just one form of matter is observed. For Instance, In a sample of salt water, the dissolved salt particles are not visible; instead, a uniform foggy mixture is present.
The mixture of salt water is a solution.
Heterogeneous Mixture: In a heterogeneous mixture, the composition is not uniform, and there are clear and distinct phases. These are the mixtures in which two or more compounds are mixed unevenly or unequally, such as oil in water and sand in water. If we split these samples into several pieces, each piece would have a different composition.
There are two types of heterogeneous mixture :
Colloid: Small, soluble particles mixed with and suspended in another substance are called colloids. Depending on the state of the suspended particles and the material, they may have different names: emulsion, foam, aerosol,etc.
Suspension: Large, soluble particles are suspended in another substance to form suspensions, which will eventually settle. For instance, flour in water When it is initially stirred, the flour particles are suspended in the water. However, after some time, the flour will sink to the bottom.
Separation Of Mixtures
The procedure or approach to separate the particles from the mixture is known as the separation of mixtures. The type of mixture and the differences in the chemical characteristics of the particles in the mixes determine which separation procedures should be implemented. There are various physical techniques that can be used to separate the components of homogeneous and heterogeneous mixtures.
Separation of Heterogeneous Mixture
Handpicking
This process basically entails hand-picking out all the undesirable materials and isolating them from beneficial ones. The separated substances might be an impurity that has to be discarded or they might both be beneficial. For instance, if you separate green grapes from black grapes in a mixture.
Threshing
Typically, this technique is used for harvesting crops. Once the wheat has been harvested, the stalks are often dried. The dried grains are then shaken off of the dry stalks and separated from the grain before being pounded into the earth.
Winnowing
Winnowing is the process of using the wind to separate grain husk from grain. Husk weighs less than wheat grains, which are heavier. A winnowing basket is used for the process of winnowing. The farmer repeatedly shakes his winnowing basket while standing on a platform that is higher above the ground, causing the mixture of wheat grains and husk to fall from a height.
Wheat grains form a pile on the ground when they fall vertically to the ground due to their weight. Because husk fragments are lighter, the wind may carry them farther. The outcome is that the husk separates from the wheat grain heap and forms its own heap. The husk and wheat grains are separated in this way.
Sieving
A shallow container called a sieve has tiny holes at the bottom. Sometimes a sieve can also be made of iron mesh. Using a sieve to divide a mixture is known as sieving. Solid mixes with components of various sizes can be separated via sieving.
A sieve is used to strain the mixture, which includes parts of various sizes, and it is repeatedly swung back and forth. Larger mixture components become stuck in the sieve because they cannot fit through the small holes in the sieve.
Magnetic Separation
Magnetic separation is the process of separating the two elements of a heterogeneous combination. Only when one of the components (like iron) has magnetic characteristics and the rest don’t can it be employed.
Sedimentation
The process of sedimentation is when heavier contaminants in a liquid, usually water, fall to the bottom of the container carrying the combination. The process requires some time to complete.

Separation of Solutions
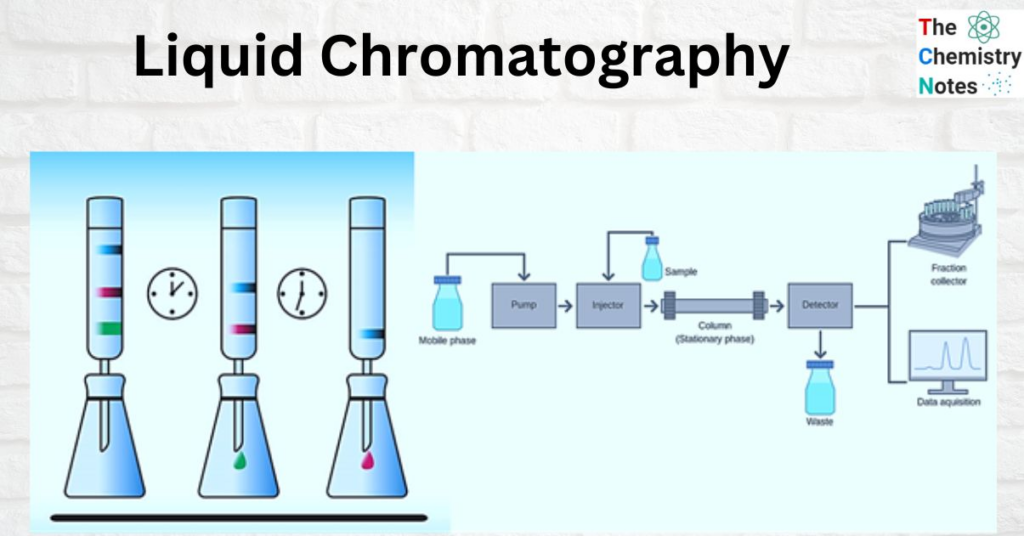
Chromatography
The process of separating a combination of components by passing them through a medium in suspension, solution, or as a vapor while moving at various rates is known as chromatography.It is a laboratory technique for the separation of a mixture into its components.
This method relies on the different characteristics of the chemicals present in the mobile and stationary phases. There are several distinct kinds of chromatographic methods, including column, TLC, paper, and gas chromatography.
Evaporation
The process of evaporation is used to separate mixtures, most frequently a mixture of a solvent and a soluble material. This approach involves heating the solution until the organic solvent turns into a gas and mainly displaces the solid residue.
To separate homogeneous mixtures with one or more dissolved salts, evaporation is a common technique. The technique divides the components into liquid and solid parts. The procedure usually involves heating the mixture until no liquid is present. Before applying this procedure, the combination should only comprise one liquid component, unless it is not required to isolate the liquid components. This is because all liquid components eventually evaporation. A soluble solid can be effectively separated from a liquid via evaporation.
![Evaporation [Image source: BBC]](https://scienceinfo.com/wp-content/uploads/2023/05/image-118.png)
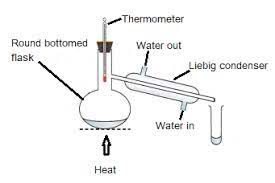
Distillation
A quick method for separating mixtures of two or more pure liquids is distillation. Distillation is a process of purification that includes vaporizing a liquid mixture’s components, then condensing and separating them. In simple distillation, a mixture is heated to a point where the component that is most volatile vaporizes. The vapor passes through a condenser, which is a cooled tube, and then condenses back into liquid. Distillate is the name given to the gathered condensate.
It is also possible to utilize other, more intricate distillation assemblies, especially to separate mixtures of pure liquids with comparable boiling points.
A simple distillation process applies to separate acetone and water. Fractional distillation is employed in separation of different fractions from petroleum products and mixture of methanol and ethanol.
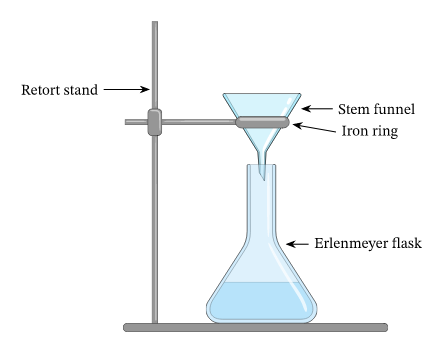
Filtration
The filtration process is the most popular way to separate a liquid from an insoluble material. Consider the concoction of sand and water as an illustration. Here, solid particles in the liquid are removed from it through filtration. Typically, various filtering agents such as filter paper or other materials are utilized.
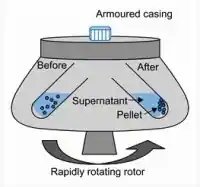
Centrifugation
Centrifugation is a technique used for the separation of tiny solid particles from a liquid that can easily pass through a filter paper. Centrifugation is used for carrying out the separation of these insoluble particles where normal filtration fails to work well. The centrifugation depends upon the viscosity of the medium, speed of rotation, shape, size, and density of the particle.
The aforementioned methodology is founded upon the fundamental concept that particles with lower mass exhibit a tendency to remain situated towards the uppermost region, while particles with greater mass or higher density are compelled to relocate towards the lowermost region as a result of swift rotation. The apparatus used for the centrifugation technique is called a centrifuge. The centrifuge mainly includes a centrifuge tube holder called a rotary. The apparatus accommodates centrifuge tubes that are evenly filled with mixtures comprising solid and liquid components in equal proportions.
Centrifugation is a commonly employed technique in clinical laboratories for conducting diagnostic analyses of blood and urine samples. Additionally, it finds application in both household and commercial settings, specifically in the separation of butter from cream in dairies and homes.This processis employed within washing machines to facilitate the extraction of moisture from damp garments.
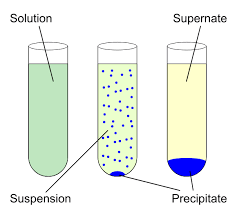
Precipitation
When two liquids are mixed together in chemistry, if the result is not soluble, a precipitate is made. In other words, a solid is made inside of a solution. For instance, when silver nitrate and silver chloride are mixed together, a deposit forms.
References
- https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/2.10/primary/lesson/methods-for-separating-mixtures-chem/
- https://amrita.olabs.edu.in/?brch=2&cnt=1&sim=96&sub=73
- https://www.geeksforgeeks.org/separation-of-mixtures/
- https://chemistryclinic.co.uk/1-10-separation-techniques/
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/mixtures-and-solutions/
