Interesting Science Videos
Saturated Hydrocarbons Definition
Saturated hydrocarbons are the simplest forms of hydrocarbons consisting entirely of single bonds that remain saturated with hydrogen atoms.
- The general formula for acyclic saturated hydrocarbons or alkanes is CnH2n+2. The more general formula can also be written as CnH2n+2(1-r), where r is the number of rings.
- Saturated hydrocarbons do not have any double or triple bonds between the carbon atoms in the structure, and all carbon atoms make four distinct covalent bonds.
- The term saturated indicates the saturation of hydrogen atoms in the structure which also makes them the simples and least polar organic compounds.
- Saturated hydrocarbons can occur either in a linear form or a branched form, depending on the complexity of the structure.
- These hydrocarbons primarily occur in petroleum products as well as other forms of fossil fuels.
- Saturated hydrocarbons burn with a blue, non-sooty flame which is why these are used as sources of fuel in vehicles and other engines.
- Substitution reaction is the characteristic property of saturated hydrocarbons as they resist other reactions like hydrogenation and oxidative addition.
- Saturated hydrocarbons have a lower concentration of carbon atoms as the number of hydrogen atoms is relatively high as compared to unsaturated hydrocarbons.
- These are also more stable and less reactive and can resist attacks by nucleophiles and electrophiles.
- Saturated hydrocarbons consist of two groups of hydrocarbon; acyclic alkanes and cycloalkanes.
- Some examples of saturated hydrocarbons include methane, butane, propane, cyclohexane, etc.
Unsaturated Hydrocarbons Definition
Unsaturated hydrocarbons are the group of hydrocarbons composed of double or triple bonds between the carbon atoms.
- Unsaturated hydrocarbons with double bonds are called alkene, which has the general formula, CnH2n, whereas those with triple bonds are called alkynes which have the general formula, CnH2n-2.
- The term unsaturated indicates that more hydrogen atoms can be added to the molecules to make them saturated.
- Like saturated hydrocarbons, unsaturated hydrocarbon can also exist in straight-chain linear form as well as branched and aromatic forms.
- All aromatic hydrocarbons are unsaturated hydrocarbons as the aromatic rings are formed by the delocalization of double bonds between multiple carbon atoms.
- Unsaturated hydrocarbons are comparatively more reactive, except aromatic compounds, and undergo multiple addition reactions on multiple bonds.
- Unsaturation of hydrocarbons can be expressed in terms of the degree of unsaturated, which is the measure of the number of π-bonds present in a molecule.
- Unsaturated hydrocarbons have a higher carbon concentration than saturated hydrocarbons as the number of hydrogen atoms is less.
- The combustion of unsaturated hydrocarbons produces a yellow flame as these have a lesser hydrogen content which, in turn, decreases the moisture of the flame.
- Unsaturated hydrocarbons, like most hydrocarbons, are non-polar, and thus, the structures are held together by weak van der Waal’s force of attraction.
- The nomenclature of unsaturated hydrocarbons is different from saturated hydrocarbons as these require the addition of numbers between the prefix to indicate the position of the double or triple bonds.
- Some examples of unsaturated hydrocarbons include ethane, acetylene, benzene, butadiene, etc.
10 Major Differences (Saturated vs Unsaturated Hydrocarbons)
| Characteristics | Saturated Hydrocarbons | Unsaturated Hydrocarbons |
| Definition | Saturated hydrocarbons are the simplest forms of hydrocarbons consisting entirely of single bonds that remain saturated with hydrogen atoms. | Unsaturated hydrocarbons are the group of hydrocarbons composed of double or triple bonds between the carbon atoms. |
| Bonds | Saturated hydrocarbons are composed of all single bonds between the carbon atoms. | Unsaturated hydrocarbons are composed of one or more double and triple bonds. |
| Concentration of carbon | Saturated hydrocarbons have a higher concentration of carbon atoms. | Unsaturated hydrocarbons have a lower concentration of carbon atoms. |
| Concentration of hydrogen | The number of hydrogen atoms is higher. | The number of hydrogen atoms is lower. |
| Reactivity | Saturated hydrocarbons are comparatively less reactive. | Unsaturated hydrocarbons are comparatively more reactive. |
| Combustion | Combustion of saturated hydrocarbons produces a blue, non-sooty flame. | Combustion of unsaturated hydrocarbons produces a yellow, sooty flame. |
| Includes | Saturated hydrocarbons include compounds like alkanes and cycloalkanes. | Unsaturated hydrocarbons include compounds like alkenes, alkynes, and aromatic compounds. |
| Reaction | Substitution reactions are the characteristic property of saturated hydrocarbons. | Addition reactions are the characteristic property of unsaturated hydrocarbons. |
| Obtained from | Saturated hydrocarbons can be obtained from fossil fuels as well as animal matter. | Unsaturated hydrocarbons are obtained from plant sources. |
| Examples | Some examples of saturated hydrocarbons include methane, butane, propane, cyclohexane, etc. | Some examples of unsaturated hydrocarbons include ethane, acetylene, benzene, butadiene, etc. |
Examples of Saturated Hydrocarbons
Methane
- Methane is the simplest aliphatic hydrocarbon consisting of a single carbon atom singly bonded to four hydrogen atoms.
- It is a saturated hydrocarbon with all four covalent bonds linked to four distinct hydrogen atoms with the molecular formula CH4.
- Methane is a simple hydrocarbon that burns with a pale, slightly luminous blue flame. It is quite stable and less flammable than other similar compounds.
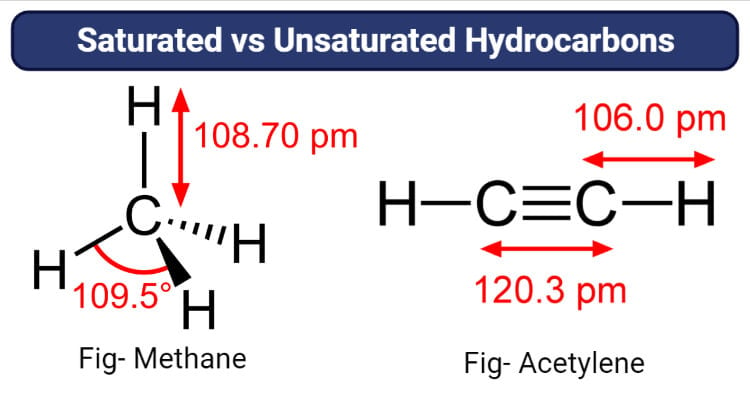
- All the four carbon-hydrogen bonds in methane are equivalent to the bond length of 1.09×10-10 and the bond angle of 109.5°. It is a tetrahedral molecule with three sp3 hybridized orbitals.
- Methane is an important fossil fuel commonly used as a form of biogas for cooking. It is also an important member of the greenhouse gases and is considered one of the prime gases involved in global warming.
Examples of Unsaturated Hydrocarbons
Acetylene
- Acetylene or ethyne is a two-carbon unsaturated hydrocarbon with the molecular formula C2H2.
- It is an alkyne with a triple bond between the carbon atoms and a single covalent bond between each carbon and hydrogen atom.
- The carbon-carbon triple bond results in a linear structure of the molecule as it places all four atoms in the same straight line with a bond angle of 180°.
- One of the triple bonds is a σ-bond, whereas the two other bonds are weaker π-bonds.
- About 20% of the total acetylene occurring in the environment is the result of oxyacetylene gas welding and cutting.
- The carbon-carbon triple bond in acetylene has energy richness which enables it to be used as a suitable substrate for bacteria.
References
- Gautam AD, Pant M and Adhikari NR (2017). Comprehensive Chemistry Part 2. Heritage Publishers and Distributors. Kathmandu, Nepal.
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 297, Methane” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Methane. Accessed 21 February, 2021.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6326, Acetylene. https://pubchem.ncbi.nlm.nih.gov/compound/Acetylene. Accessed Apr. 8, 2021.
- https://pediaa.com/difference-between-saturated-and-unsaturated-hydrocarbons/
