In 1911, Rutherford and his assistants, H. Geiger, and E. Marsden, conducted the Alpha Particle scattering experiment, which resulted in the formation of the ‘nuclear model of an atom.’ α-ray Scattering Experiment was a successful attempt to present a Rutherford atomic model. In this experiment, the highly energetic alpha particles obtained from the radioactive substance known as radium are allowed to pass through the very thin sheet of gold foil (100 nm thickness). The α-particles are deflected, and they collide with the fluorescent zinc sulfide screen, where tiny flashes of light can be seen using a moving microscope. Rutherford derived various conclusions from this alpha-ray scattering experiment that contradict Thomson’s Atomic Model.
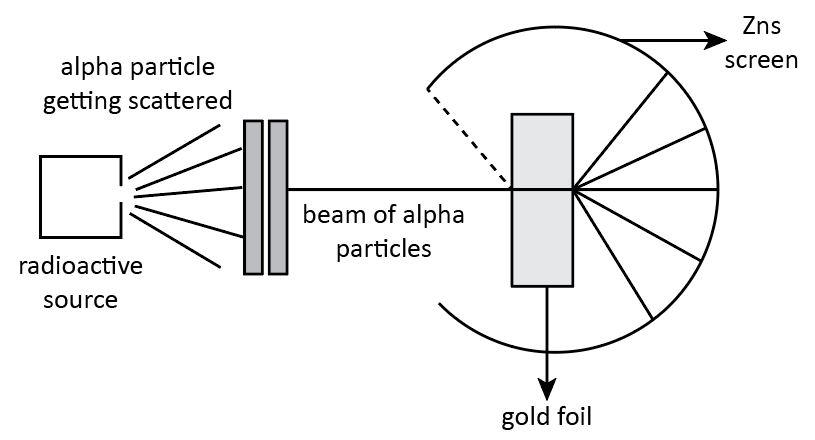
Rutherford’s Alpha Ray Scattering Experiment
Image source: https://www.vedantu.com/question-answer/rutherfords-alpha-particle-scattering-experiment-class-10-chemistry-cbse-5f8dd7c6f40b651ca5561dd8
Observations of Rutherford’s α-ray Scattering Experiment
Since α -particles are highly energetic, it was assumed that they would pass through the gold foils. Additionally, if the mass and positive charge are distributed uniformly across the metal, alpha particles will pass through the gold foils without any deflections or deviations. However, Rutherford discovered some unexpected findings, which are shown below:
The α-particles are very energetic substances, so it was thought that they would pass through the gold foils. Alpha particles would go through the metal without undergoing any kind of deflections or deviations if the mass and the positive charge are distributed uniformly throughout the metal. However, Rutherford observed some unexpected results which are given below:
I) A major fraction (about 99%) of the α-particles pass through the gold foil without undergoing any kind of deflections or deviations.
II) A few of the α-particles were deflected from their original path by very small angles.
III) A very small number of α-particles were deflected back and returned towards the same path.
Conclusions Drawn from Rutherford’s α-ray Scattering Experiment
Rutherford deduced the following conclusions based on the observation of the α-ray scattering experiment:
I) The majority of alpha particle passes through the gold foil without any deflections means that most of the space in an atom is empty.
II) Some of the alpha particles undergo small deviations, this shows that the atom contains a positive charge which is not distributed uniformly in an atom. The positive charge concentration is very small in an atom.
III) Very few alpha particles were turned on its path which suggests that the total volume occupied by the positively charged particles i.e. nucleus is very small in comparison to the total volume occupied by an atom.
Rutherford Atomic Model
Based on the observations and conclusions drawn from the α-ray scattering experiment, Rutherford proposed a model of the atom which is named after him as the Rutherford’s Nuclear Model of Atom. The main postulates according to the Rutherford atomic model are as follows:
I) An atom has a tiny dense center core that contains the whole mass of the atom, leaving the rest of the atom practically empty. The positive charge and the majority of the mass are concentrated in a small region known as the nucleus. The nucleus (in a range of 10-15 m) is exceedingly small compared to the atom (in the scale of 10-10 m).
II) As the atom is electrically neutral, the number of protons inside the nucleus equals the number of electrons moving around it.
III) Negatively charged electrons revolve in a predetermined circular path around the nucleus. Rutherford named this circular route an orbit or shell.
IV) Electrons revolve around the nucleus in the fixed circular path as the electrostatic force of attraction between the electrons and the nucleus is balanced by the outward centrifugal force due to the circular motion of the electrons around the nucleus. Thus the obtained atomic model is considered a planetary atomic model.
This can be shown by the formula:
mv2/r = kq1q2/r2
Drawbacks of Rutherford’s Atomic Model
Although the Rutherford atomic model was based on experimental observations, some serious objections against this model were reported such as:
I) The atomic model fails to explain atom stability.
II) This atomic model also fails to explain the presence of distinct lines in the hydrogen spectrum.
III) Particles traveling in a circular direction may experience some acceleration and emit some energy. Thus, an electron circulating in a circular path would lose energy and eventually fall into the nucleus.
IV) The electrons’ configuration along a circular channel was not specified.
suggested video:
References
- Lee, J.D. (1996) Concise Inorganic Chemistry. 5th Edition, Chapman and Hall Ltd., London, 619-621.
- A New Comprehensive Chemistry Grade XI by Prof.Dr.P Wagley.
- https://vixra.org/pdf/1805.0246v1.pdf.
- https://122.physics.ucdavis.edu/sites/default/files/files/Rutherford/rutherford122%202.pdf.
- https://www.vedantu.com/iit-jee/rutherford-scattering-experiment.
- https://byjus.com/chemistry/rutherfords-model-of-atoms-and-its-limitations/#:~:text=Rutherford’s%20Alpha%20Scattering%20Experiment,-Rutherford%20conducted%20an&text=Rutherford%2C%20in%20his%20experiment%2C%20directed,around%20the%20thin%20gold%20foil.
