Ruthenium is a metallic element with the atomic number 44 and is represented by the symbol ‘Ru’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 8 of the periodic table. It is one of the rare transition metals that belongs to the platinum group of the periodic table.
Ruthenium is a relatively rare metal that is hard, glossy, brittle, and silvery-white at room temperature. Its concentration on the Earth’s crust is roughly 100 parts per trillion, thus making it the 78th most prevalent element on the crust. It occurs naturally as a free element, frequently in conjunction with other platinum-group metals. It’s commercially obtained from pentlandite (an iron-nickel sulfide that contains trace amounts of ruthenium).
History of Ruthenium
- Pre-Columbian Americans employed platinum alloys containing ruthenium for a long time, and European chemists utilized it as a material around the mid-1500s without knowing its elemental composition.
- In the year 1807, Polish chemist Jedrzej Sniadecki claimed to have isolated element 44 from South American platinum ores. He announced the discovery of the element and named it “vestium” in 1808, but he withdrew his claim of discovery later.
- In the year 1828, Swedish chemist Jons Jacob Berzelius and Russian chemist Gottfried W. Osann investigated the residue that was left after the reduction of crude platinum ores in aqua regia.
- Osann was convinced that three new elements were left in the residue,one of which he named ruthenium.
- In the year 1844, a Russian chemist, Karl Karlovich Klaus, discovered ruthenium while he was analyzing the residue from a sample of platinum ores from the Ural Mountains.
- Ruthenium derives from the Latin word ‘Ruthenia’, which was the old name used for the land of Russia.
Occurrence of Ruthenium
- Ruthenium is one of the rarest metals. Its presence on the Earth’s crust is roughly 0.001 parts per trillion, which makes it the 78th most prevalent element on the crust.
- Ruthenium is usually found in platinum ores. It is also found in pentlandite ores (Fe, Ni)9S8 and pyroxenite rocks.
- Ruthenium is obtained commercially as a by-product from copper,and platinum ore processing. It is also obtained as a byproduct of the Nickel mining operation in the Sudbury region of Ontario, Canada.
Isotopes Of Ruthenium
Ruthenium has seven naturally occurring stable isotopes; 96Ru, 98Ru, 99Ru, 100Ru, 101Ru, 102Ru, and 104Ru.
Naturally occurring isotopes of Ruthenium
| Isotope | Natural abundance (atom %) |
|---|---|
| 96Ru | 5.54 (14) |
| 98Ru | 1.87 (3) |
| 99Ru | 12.76 (14) |
| 100Ru | 12.60 (7) |
| 101Ru | 17.06 (2) |
| 102Ru | 31.55 (14) |
| 104Ru | 18.62 (27) |
Elemental Properties of Ruthenium
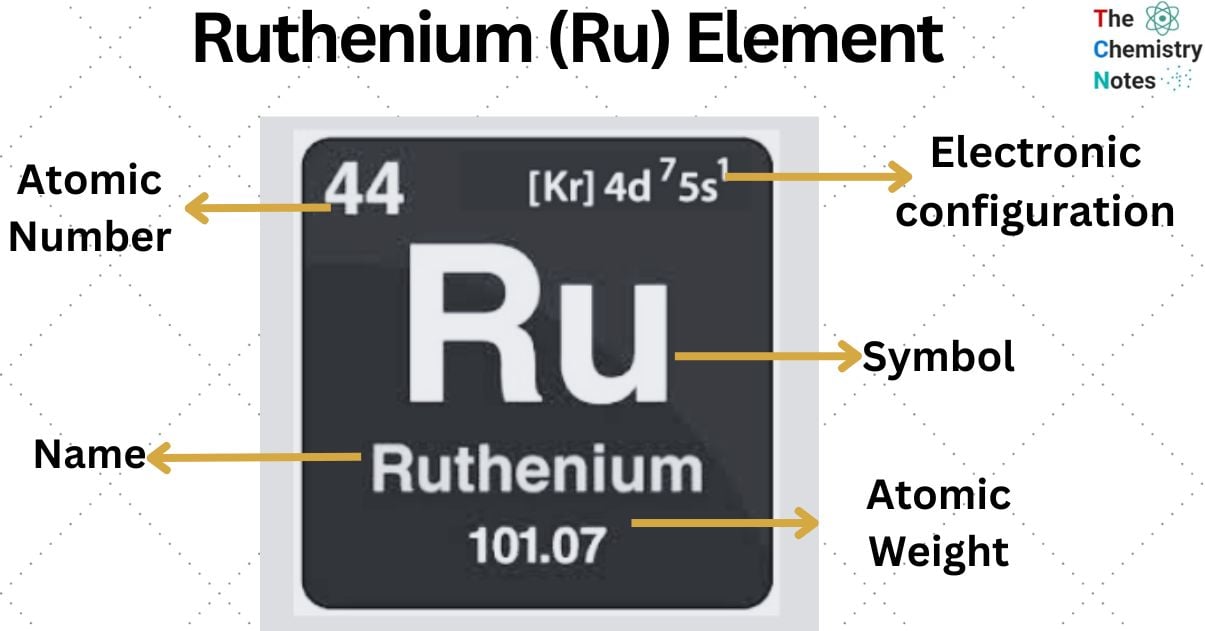
| Electronic Configuration | [Kr] 4d7 5s1 |
| Atomic Number | 44 |
| Atomic Weight | 101.07 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 8, 5, d-block |
| Density | 12.45 g.cm -3 at 20 °C |
| Van der Waals radius | 0.135 nm |
| Electron shells | 2, 8, 18, 15, 1 |
| Electrons | 44 |
| Protons | 44 |
| Neutrons in most abundant isotope | 58 |
Physical Properties of Ruthenium
- Ruthenium has an atomic number of 44 and is a silvery-white metal. It has a melting point of 2334 °C (4233 °F) and a boiling point of 4150 °C (7502 °F).
- Ruthenium has a solid phase density of 12.45 gm/cm3 and a liquid or molten phase density of 10.65 gm/cm3.
- It is recognized as a ferromagnetic element because of its unusual crystalline structure and internal electronic configuration; it becomes magnetic once subjected to an external magnetic field. It exhibits ferromagnetism at room temperature, including that of three other elements: iron, cobalt, and nickel.
- Ruthenium is malleable, allowing it to be easily hit into sheets without cleavage, and ductility, which makes it possible to draw thin wires from it.
- Ruthenium has the property of high inherent tensile strength, which makes it easier to shape it. Because of the tensile strength iron has it can provide solidity to structures, which makes it even more popular. It has a high tensile strength of 53700 psi.
- Ruthenium is sonorous meaning it can make a sound when it is struck with some other object.
- Ruthenium is an effective thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Ruthenium serves as an excellent electrical conductor. Because electrons in ruthenium are free to move around they are able to carry electrical charge from one end to other.
| Color/physical appearance | Lustrous, silvery, white |
| Melting point/freezing point | 2607 K (2334 °C, 4233 °F) |
| Boiling point | 4423 K (4150 °C, 7502 °F) |
| Density | 11.45 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 2.2 (Pauling Scale) |
Chemical Properties of Ruthenium
- Ruthenium does not tarnish at room temperature when exposed to the air.
- Ruthenium oxidizes when heated above 800°C.
- It dissolves in fused alkali, which further does not react with the aqua regia.
- Ruthenium reacts with halogens.
Chemical Reaction of Ruthenium
- The Reaction Of Ruthenium with Water
Ruthenium does not react with water under normal conditions.
- The Reaction Of Ruthenium with Air
Ruthenium does not react with oxygen at room temperature, however when heated above 800°C it oxidizes to form ruthenium (IV) oxide, RuO2.
Ru (s) + O2 (g) → RuO2 (s)
- The Reaction Of Ruthenium with Halogens
Ruthenium reacts with an excess of fluorine, F2, to form ruthenium(VI) fluoride, RuF6.
Ru (s) + 3 F2 (g) → RuF6 (s) [dark brown]
When heated at 330°C, in the presence of carbon monoxide (CO), ruthenium reacts with chlorine, Cl2, to produce dark brown ruthenium (III) chloride, RuCl3. If it is heated even more it forms a black form of ruthenium (III) chloride.
2 Ru (s) + 3 Cl2 (g) → 2 RuCl3 (s)
Uses Of Ruthenium
Ruthenium has important physical and chemical properties which can make it highly applicable in different industries. some of the important applications of metal are discussed below:
Used In Alloy: When ruthenium is added in small quantities to palladium, platinum, and titanium alloys, it increases the corrosion resistance and durability of the alloys. This characteristic helps in the production of palladium jewelry. When ruthenium forms an alloy with titanium, it increases the corrosion resistance, which is further used for the manufacturing of jet engine turbines. Medical equipment and devices for measuring extreme temperatures are usually made from ruthenium alloys.
Used As Catalyst: Ruthenium is used as a catalyst for homogeneous and heterogeneous reactions. A catalyst is a substance that enhances the reaction without changing itself. They are generally used for the production of drugs and advanced materials. It can also be used to transform photoelectric energy into electrical energy.
Used In Micro-Electronics: Ruthenium compounds have the potential to replace other metals and silicides in microelectronic components. Also, ruthenium oxides are useful because their characteristics enable them to be compatible with the semiconductor processing methods needed to make microelectronic components.
Used in Medical Treatment: Ruthenium has also been used in medical procedures. A common immunosuppressant called Cyclosporin A causes adverse effects like nausea, renal problems, and hypertension. It is used to treat conditions like anemia and psoriasis eczema. Complexes containing Ru(III) are created to change Cyclosporin A’s mechanism of action. A stable chemical that has an inhibitory effect on T lymphocyte proliferation is produced by the ruthenium cyclosporin complex.
Health Effects of Ruthenium
- Ruthenium is rare and toxic, so all ruthenium compounds should be handled carefully as they are carcinogenic.
- When mishandled the ruthenium compounds can leave a noticeable mark on the skin.
- It suggests that ruthenium is completely preserved in bones after ingestion.
- Ruthenium oxide, RuO4, is highly poisonous and volatile and should be avoided.
Environmental Effects Of Ruthenium
Although extensive research has yet to be done, algae seem to have concentrated ruthenium.
There have been no negative impacts of ruthenium on the environment proven yet.
References
- https://www.chemicool.com/elements/ruthenium.html
- Mary Elvira Weeks, The Discovery of the Elements. VIII. The Platinum Metals., J. Chem. Educ., June 1932, p1028-1032.
- https://www.lenntech.com/periodic/elements/ru.htm
- Matthey, Johnson. “The Discovery of Ruthenium”. Johnson Matthey Technology Review. Retrieved 25 August 2020.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN0-8493-0486-5. Section 11, Table of the Isotopes
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
- https://www.intechopen.com/chapters/61475

