Reverse-phase chromatography (RPC) is a liquid chromatography technique that involves the separation of molecules based on their hydrophobicity. It is a type of high-performance liquid chromatography (HPLC) that uses a polar mobile phase made up of water and at least one organic solvent and a nonpolar stationary phase to separate samples.
Adsorption chromatography is another name for reverse-phase chromatography, which represents an adsorptive procedure based on a partitioning mechanism of separation.
Both analytical and preparative applications of reverse-phase phase chromatography have been discovered in the field of biological separation and purification. Reverse phase chromatography can separate molecules with some degree of hydrophobicity, such as proteins, peptides, and nucleic acids, with great recovery and resolution.This separation was achieved through the reversible adsorption of solute molecules with different levels of hydrophobicity.This new chromatographic mode employed a nonpolar stationary phase and a polar mobile phase, which was the opposite of the normal mode of operation. As a result, it was referred to as a “reverse” mode.
Interesting Science Videos
Principle of reverse phase chromatography
The hydrophobic binding relationship between the solute molecule in the mobile phase and the immobilised hydrophobic ligand, i.e. the stationary phase, determines the separation mechanism in reverse phase chromatography.The solute molecules distribute between the mobile phase and the stationary phase. The distribution of a solute between these two phases is determined by the medium’s binding qualities, the solute’s hydrophobicity, and the mobile phase’s composition. Adsorption of the solute from the mobile phase to the stationary phase is initially favored by the experimental setup.
Matrix used in reverse phase chromatography
The essential variables that influence the separation process in reverse-phase chromatography include the chemical composition of the matrix, particle size, type of immobilized ligand, ligand density on the surface of the stationary phase, and pore size of the matrix.
A reverse-phase chromatographic medium is made up of hydrophobic ligands that have been chemically bonded to a porous, insoluble beaded matrix. The matrix must be chemically as well as mechanically stable. Silica or a synthetic organic polymer like polystyrene typically makes up the base matrix for the commercially available reversed phase media.
The surface area of porous silica is large. Hence, a substantial number of hydrophobic chains may be linked to the support, offering high solute loading capacities. Many of the commercially available stationary phases are monomeric, with the silica support functionalized with a silanizing reagent with one hydrolyzable group.In RPLC, stationary phases consisting of a single linear aliphatic chain are most commonly utilized. The chain length (total number of carbon atoms) and bonding density determine the hydrophobicity of these stationary phases. C18 and C8 are the two most widely used RPLC materials.
As reverse-phase media, synthetic organic polymers such as beaded polystyrene are also available. The main advantage of polystyrene media is their exceptional chemical stability, especially in very acidic or basic environments. Unlike silica gels, which have hydrophobic ligands grafted to a hydrophilic surface, the surface of the polystyrene bead is extremely hydrophobic.
Additionally, Polystyrene, in contrast to silica gels, is stable at all pH ranges between 1 and 12. So, r Reverse phase separations utilizing polystyrene-based media can be conducted above pH 7.5, resulting in higher retention.
Mobile phase
The mobile phase significantly affects the solute separation process. In most cases, binary mixes of water and an organic solvent are employed, although ternary or quaternary mixtures of water and two or three different organic solvents can also be utilized to regulate the strength and selectivity of elution. The degree of interaction between the solutes and the stationary phase is determined by the mobile-phase composition, which includes solvents, buffers, and other additives. To maximize each of these elements a variety of strategies can be used. Theoretically, a large range of water-miscible organic solvents can be employed as modifiers, however, only a few of them are used in RPLC, including acetonitrile, methanol, ethanol, tetrahydrofuran, and isopropanol.
The primary objective of utilizing these organic solvents is to retain the mobile phase’s polarity low enough to dissolve the partially hydrophobic solute yet high enough to enable the solute’s binding to the reverse-phase phase chromatographic matrix. The process of changing the number of organic solvents in the mobile phase to isolate a molecule of interest is known as a gradient elution.
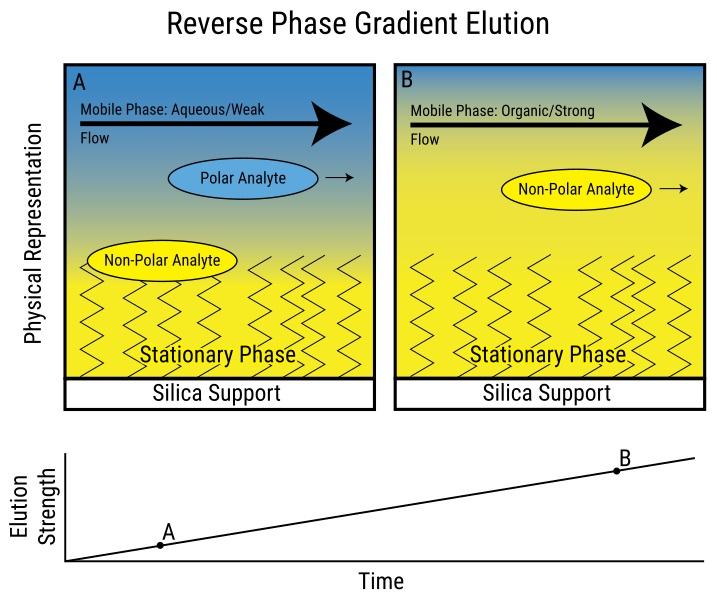
Gradient elution in reverse phase chromatography
Source: https://pediaa.com/difference-between-normal-phase-and-reverse-phase-chromatography/
Steps involved in the reverse phase chromatography
Steps for reverse-phase chromatography to be taken into consideration while performing reverse-phase chromatography include:
- First step involve the preparation of column with suitable solid support.
- Secondly, equilibrate the column filled with the reverse phase medium under suitable initial mobile phase conditions of pH, ionic strength, and polarity.
- The sample containing the desired solutes is applied to the column. The sample should ideally dissolve in the same mobile phase that is used to equilibrate the chromatographic bed. The sample is applied to the column at the proper flow rate for binding.
- After applying the sample, the chromatographic bed is further rinsed with a mobile phase to eliminate undesirable and unattached solute molecules. Then the bound solute molecules will successively desorb and elute from the column by altering the polarity of the mobile phase.
- After applying the sample, the chromatographic bed is further washed with a mobile phase to eliminate undesirable and unattached solute molecules.
- . Then by altering the polarity of the mobile phase, the bound solute molecules will successively desorb and elute from the column.
- Finally, the chemicals that have not already been desorbed are eliminated by changing the mobile phase near to 100% organic modifier to ensure full removal of all bound substances.
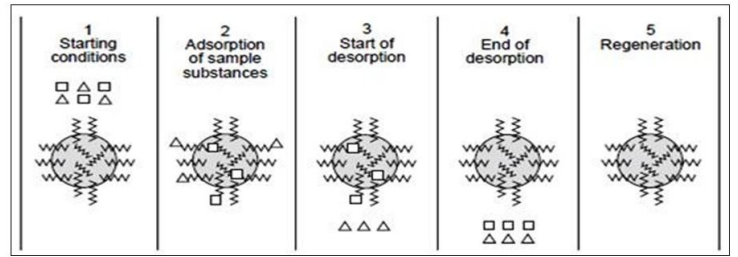
Process of reverse phase chromatography
source: https://wjpr.s3.ap-south-1.amazonaws.com/article_issue/1625307492.pdf
Separation in reverse-phase chromatography is caused by differences in the binding abilities of the solutes in the sample as a result of their hydrophobic characteristics. The initial mobile phase’s hydrophobic characteristics can be used to regulate the degree of solute molecule binding to the reversed phase medium. Although it can be challenging to quantify how hydrophobic a solute molecule is, it is simple to separate solutes with barely detectable differences in their hydrophobicity. Reverse phase chromatography is an essential technology for the high performance separation of complex biomolecules due to its excellent resolving power.
Factors affecting the separation in reverse phase chromatography
Difference factors influence the rate of the seperation process in reverse phase chromatography. They include:
I. Column length
In reverse phase separations, the resolution of large biomolecules is less susceptible to column length than the resolution of small organic molecules. Proteins, large peptides, and nucleic acids can be effectively purified on short columns, and increasing column length has no noticeable effect on resolution. Increased column length can occasionally increase the resolution of tiny peptides (including some peptides).
II. Mobile phase
In many contexts, the mobile phases in reverse phase chromatography are referred to informally as “buffers.” Although the mobile phase solutions often contain strong acids at low pH levels with high concentrations of organic solvents, they have the minimal buffering ability. While operating closer to physiological circumstances, adequate buffering capacity should be maintained.
Large molecules desorb in a highly limited range of organic modifier concentrations because the partition coefficients of high molecular weight solutes are particularly sensitive to minor changes in the mobile phase composition.
III. Organic solvents
The addition of the organic solvent (modifier) reduces the polarity of the aqueous mobile phase. In reverse phase chromatography, the mobile phase’s eluting strength increases with decreasing polarity. Acetonitrile, isopropanol, and methanol are the most commonly utilized organic modifiers. All three solvents are basically UV transparent. Since column elution is frequently observed with UV detectors, this characteristic is essential for reverse phase chromatography.
IV. Ion supression
The mobile phase pH can affect the retention of proteins and peptides in reverse phase chromatography because these particular solutes possess ionizable groups. The mobile phase’s pH will determine the level of ionization. Trifluoroacetic acid (or ortho-phosphoric acid are two common strong acids used to prepare the mobile phase for reverse phase chromatography. These acids keep the pH level low and prevent the acidic groups in the solute molecules from ionizing. The number of strong acid components in the mobile phase can affect the ionization of the solutes and hence their retention behavior.
V. Ion pairing agent
The retention periods of solutes such as proteins, peptides, and oligonucleotides can be altered by introducing ion pairing agents to the mobile phase. Ionic interactions cause ion pairing agents to bind to the solute, resulting in a change in the hydrophobicity of the solute.
Reverse phase chromatography in general practice
The most effective technique for separating and analyzing biomolecules, peptides, and proteins is reverse phase chromatography in combination with high-performance liquid chromatography. The following are the causes for this:
- The matrix’s stability in various mobile phase.
- Separations are reproducible.
- High resolutions are possible for target molecules (either closely related or structurally distinct).
- Significant recoveries and throughput.
- Gradient elution makes separations easier.
Advantages of reverse phase chromatography
- Reverse phase chromatography is an affordable procedure when compared to other chromatographic techniques and offers an accurate result with a minimal amount of sample.
- Reverse phase chromatography also offers the advantage of using pH selectivity to improve separation.
- Most organic molecules are retained by the reverse-phase column’s hydrophobic stationary phase.
- It can be used to analyze a wide range of compounds, including neutral polar and nonpolar solutes as well as acidic, basic, and amphoteric substances.
Disadvantages of reverse phase chromatography
- Pressure must be generated in reverse-phase high-performance liquid chromatography (RP-HPLC).
- Analyzing amines and compounds that are insoluble in water is more challenging.
- To confirm the identity of the analytes, additional methods are required.
- Using the system demands more technical knowledge and skills.
Advantages of reverse phase chromatography over normal phase chromatography
The reverse phase chromstogrphy mode has several advantages over normal-phase approaches in preparative separations. Some of them are as follows:
- This process removes solubility difficulties that are commonly encountered in non-polar normal phase solvents.
- Uses less hazardous solvents.
- Allows quick sample recovery.
- Provides a method of removing impurities and mobile phase additives.
Suggested video
References
- https://onlinelibrary.wiley.com/doi/abs/10.1002/9783527678129.assep008
- https://www.sciencedirect.com/science/article/abs/pii/S0076687909630093
- http://wolfson.huji.ac.il/purification/PDF/ReversePhase/AmershamRPCManual.pdf
- https://pubs.acs.org/doi/10.1021/acs.analchem.8b04699
- https://www.obrnutafaza.hr/pdf/Reversed-phase-chromatography-general-introduction-for-improved-method-development.pdf
- https://www.researchgate.net/publication/279957463_Strategies_for_Protein_Separation
