The reduction reactions involve the formation of bonds between organic compounds and hydrogen by removing unsaturated bonds or bonds to other heteroatoms.
Some reduction reactions are as follows:
Interesting Science Videos
Clemmensen reduction
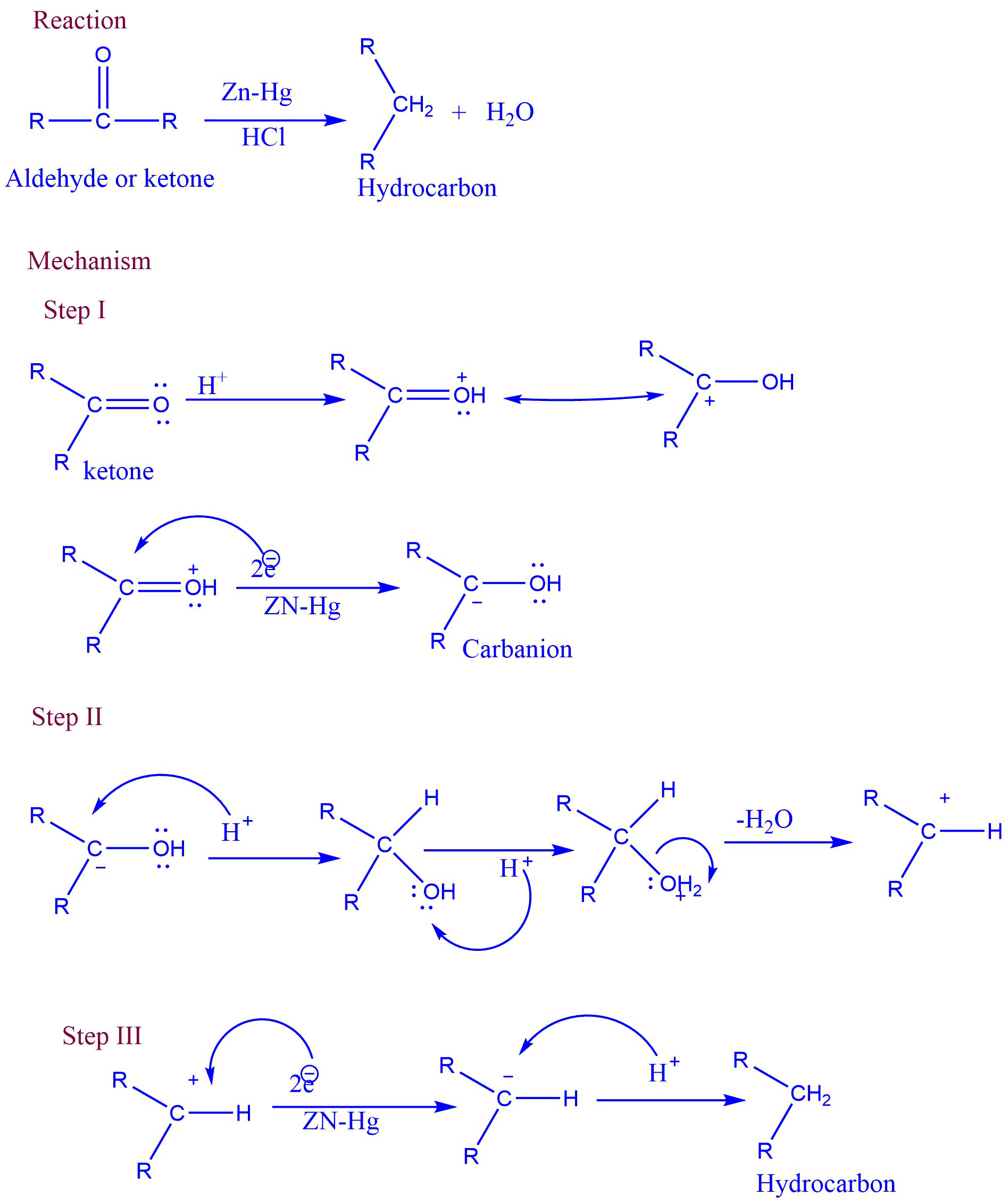
Aldehyde or ketone reacts with Zn-Hg in the presence of conc. hydrochloric acid to form the corresponding hydrocarbon. This reaction is known as Clemmensen reduction.
In this reaction, acid supplies proton to the oxygen. At the same time, metal supplies an electron pair to electron-deficient carbonyl carbon to form carbanion.
Mechanism of Clemmensen reduction
The mechanism involves three steps:
First step: Formation of carbanion
Second step: Elimination of water
Third step: Addition of proton
Apllications of Clemmensen reduction
- The reduction mechanism is commonly used to convert the carbonyl group to the methyl group.
- It is an important application in preparing polycyclic aromatic compounds containing unbranched side hydrocarbon chains.
- It is commonly used to convert acyl benzene to alkyl benzene.
Some reactions
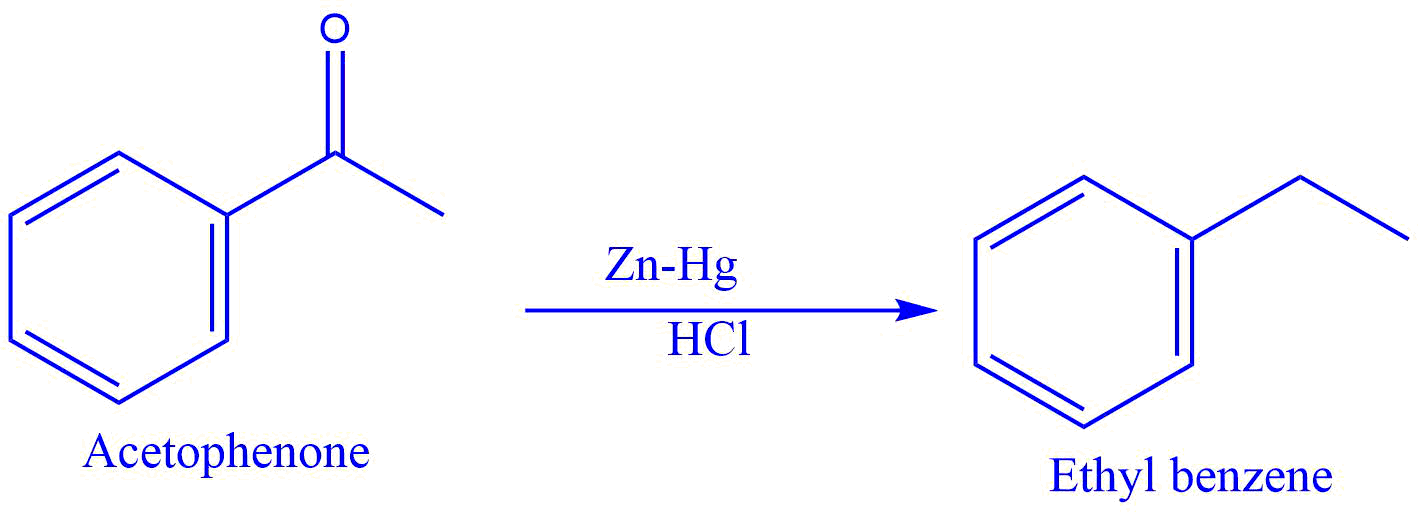
1. Conversion of acetophenone into ethyl benzene.
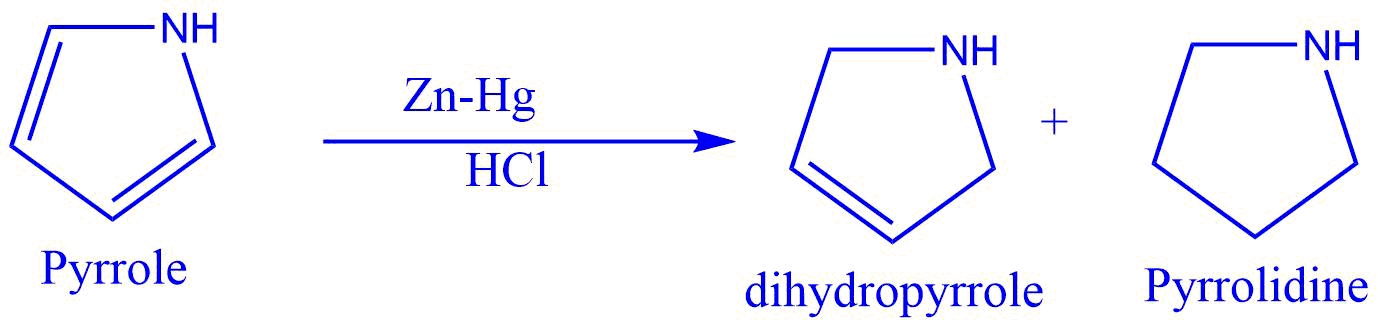
2. Conversion of pyrrole to dihydropyrrole and pyrrolidine
Wolff-Kishner reduction
In basic conditions, aldehyde and ketone react with hydrazine to produce hydrocarbon. This reaction is called Wolf kishner reduction.
Mechanism of Wolff Kishner reduction
Wolf-kishner reduction involves a three-step reaction process. These are as follows:
I. The electrophilic addition of hydrazine to C=O to produce hydrazone.
II: Addition of base
III: Removal of nitrogen
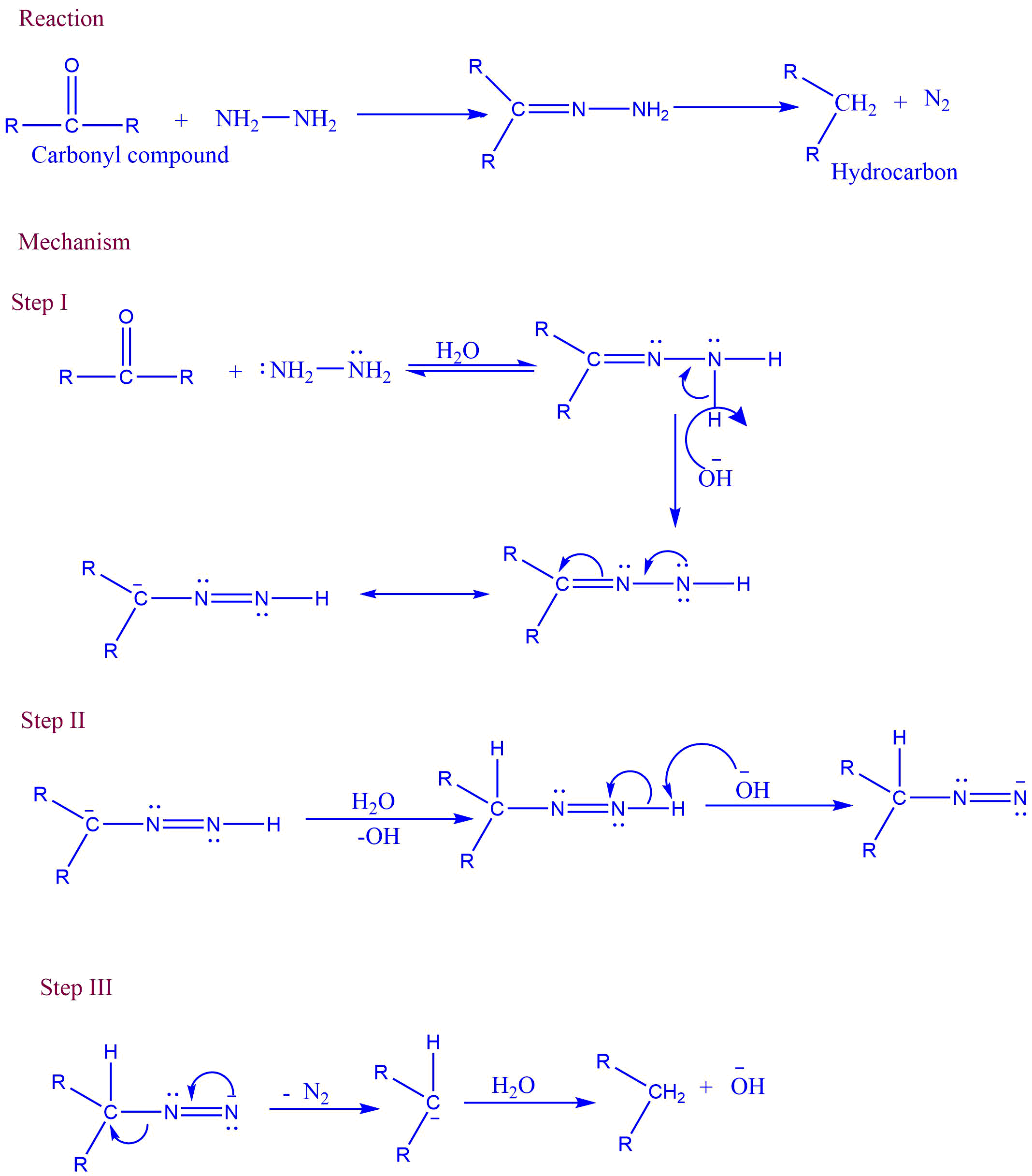
Applications of Wolff Kishner reduction
- It is usually used to convert carbonyl group (-C=O) to methylene group (-CH2)
- Synthesis of long-chain alkyl chain to the benzene ring can be carried out by using modified Wolff Kishner reduction.
Some reactions
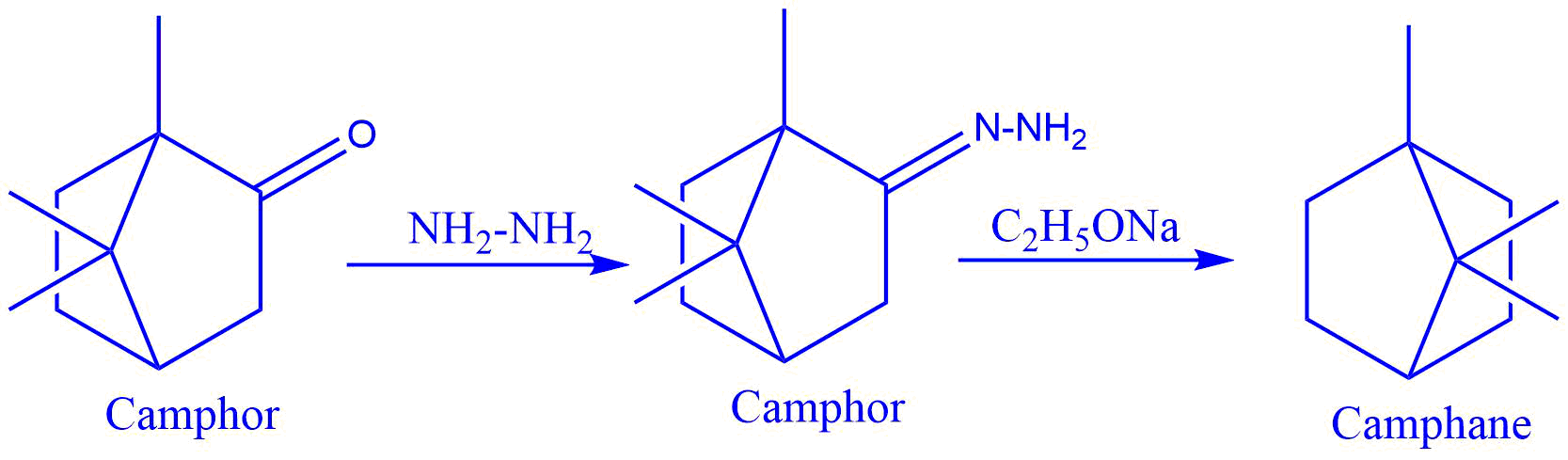
- Reduction of camphor to camphane
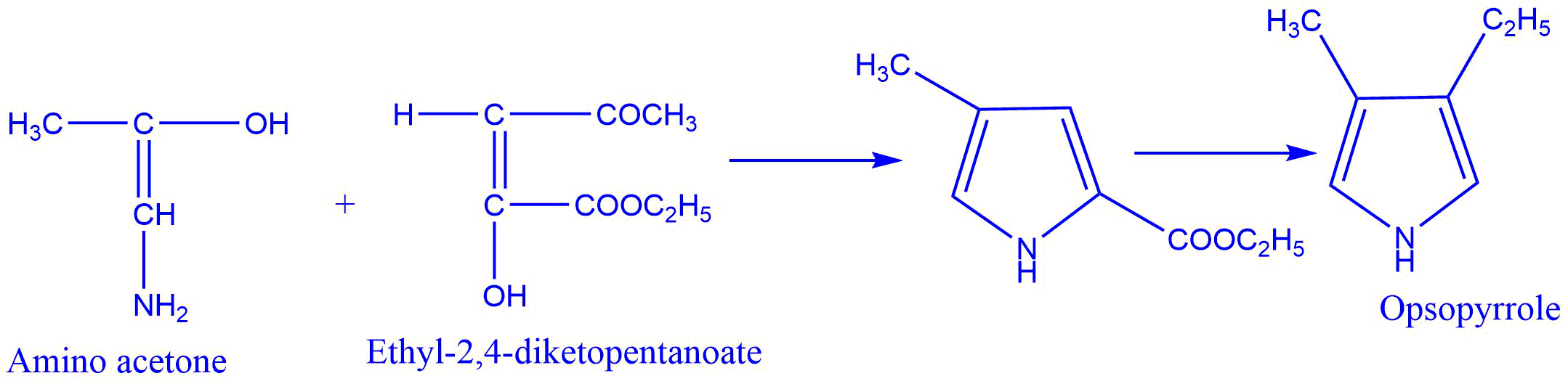
- Synthesis of pyrrole
Birch reduction
In Birch reduction, diene undergoes 1.4-addition, and arenes give nonconjugated cyclohexadiene. Here aromatic substrate undergoes reduction with alkali metals in a mixture of ammonia and alcohol.
Mechanism of Birch reduction
Step I and Step III involve the single electron transfer from metal ions, while Step II and Step IV involve the proton transfer from alcohol.
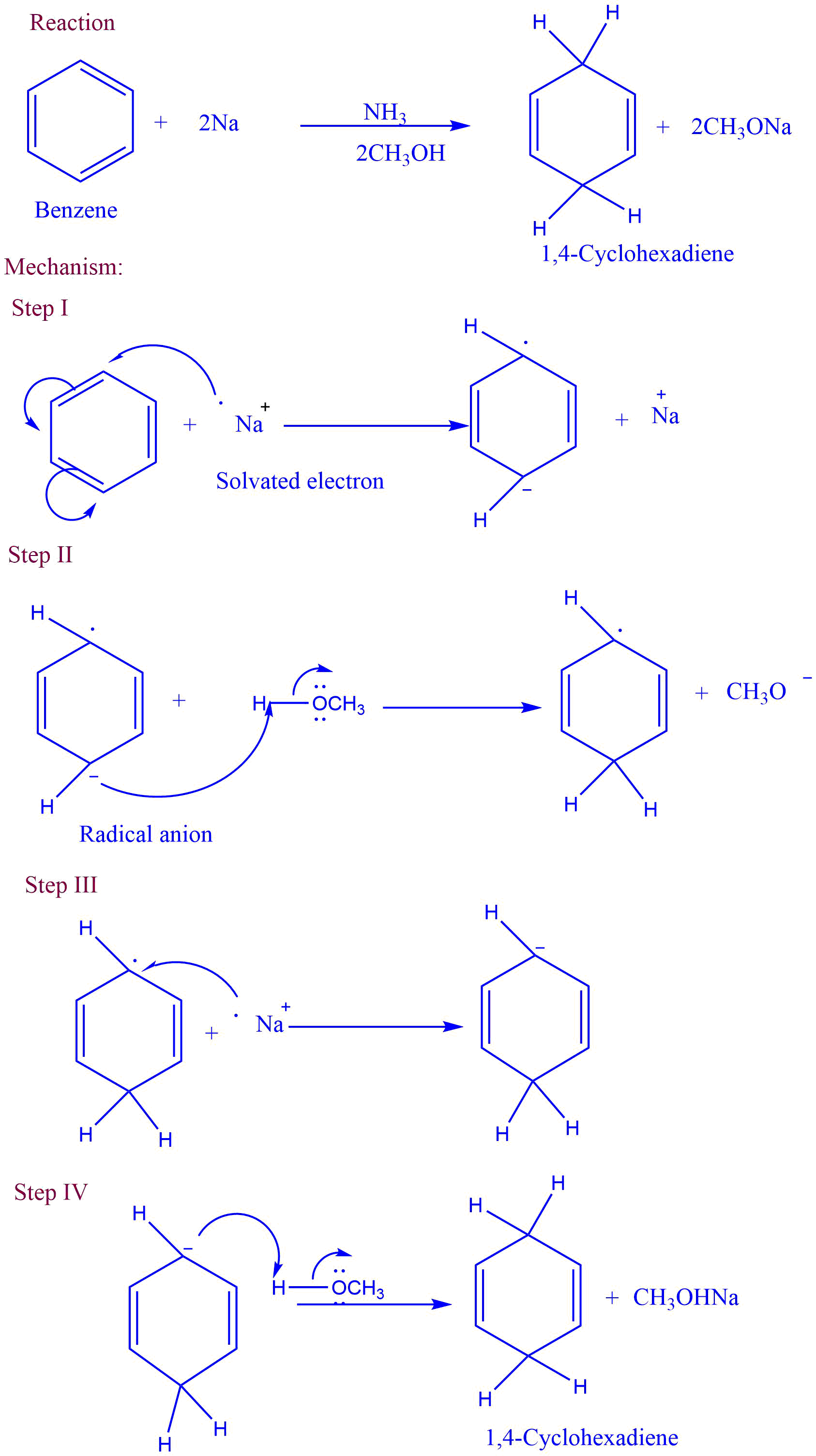
Application of Birch reduction
- It transforms aromatic compounds with benzenoid rings into 1,4 cyclohexadiene.
- This is quite unlike catalytic hydrogenation, which typically reduces aromatic rings.
Some reactions
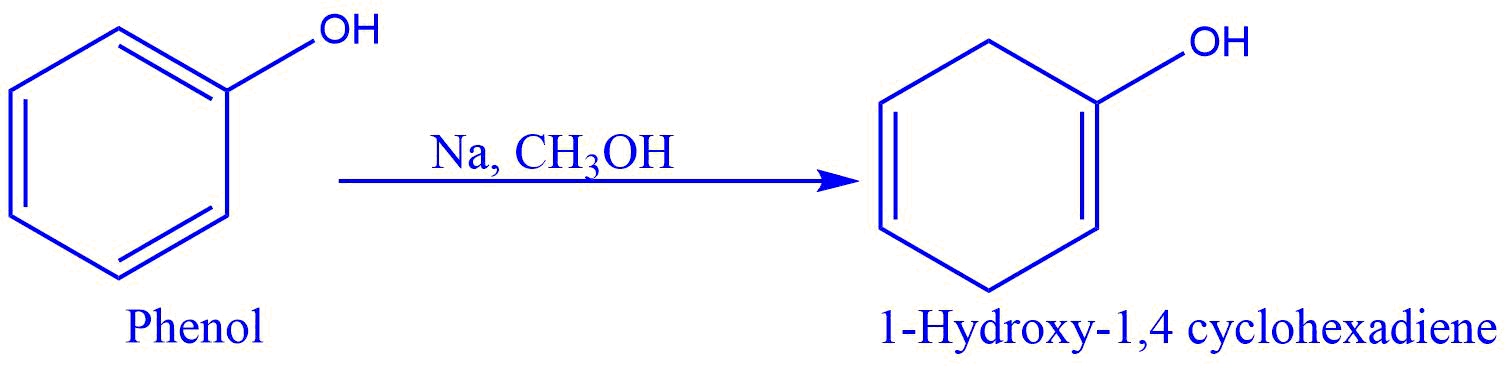
- Conversion of phenol into 1-hydroxy-1,4 cyclohexadiene
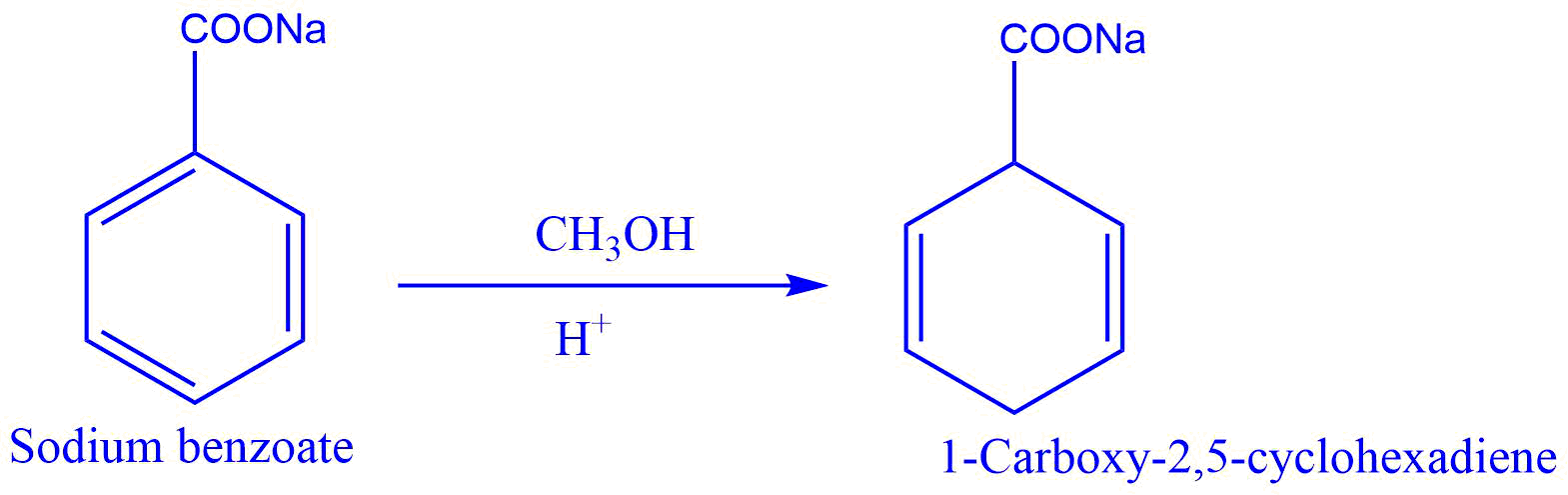
- Reduction of sodium benzoate to 1-carboxy-2,5-cyclohexadiene
Catalytic hydrogenation
Catalytic hydrogenation is the direct addition of molecular hydrogen to a double bond in the presence of a transition metal catalyst such as Ni, Pt, Pd, Ru, or their compounds.
The reaction is believed to involve chemisorption, which is the exothermic adsorption of gases by solids and involves the formation of a chemical link between the adsorbing and adsorbed materials.
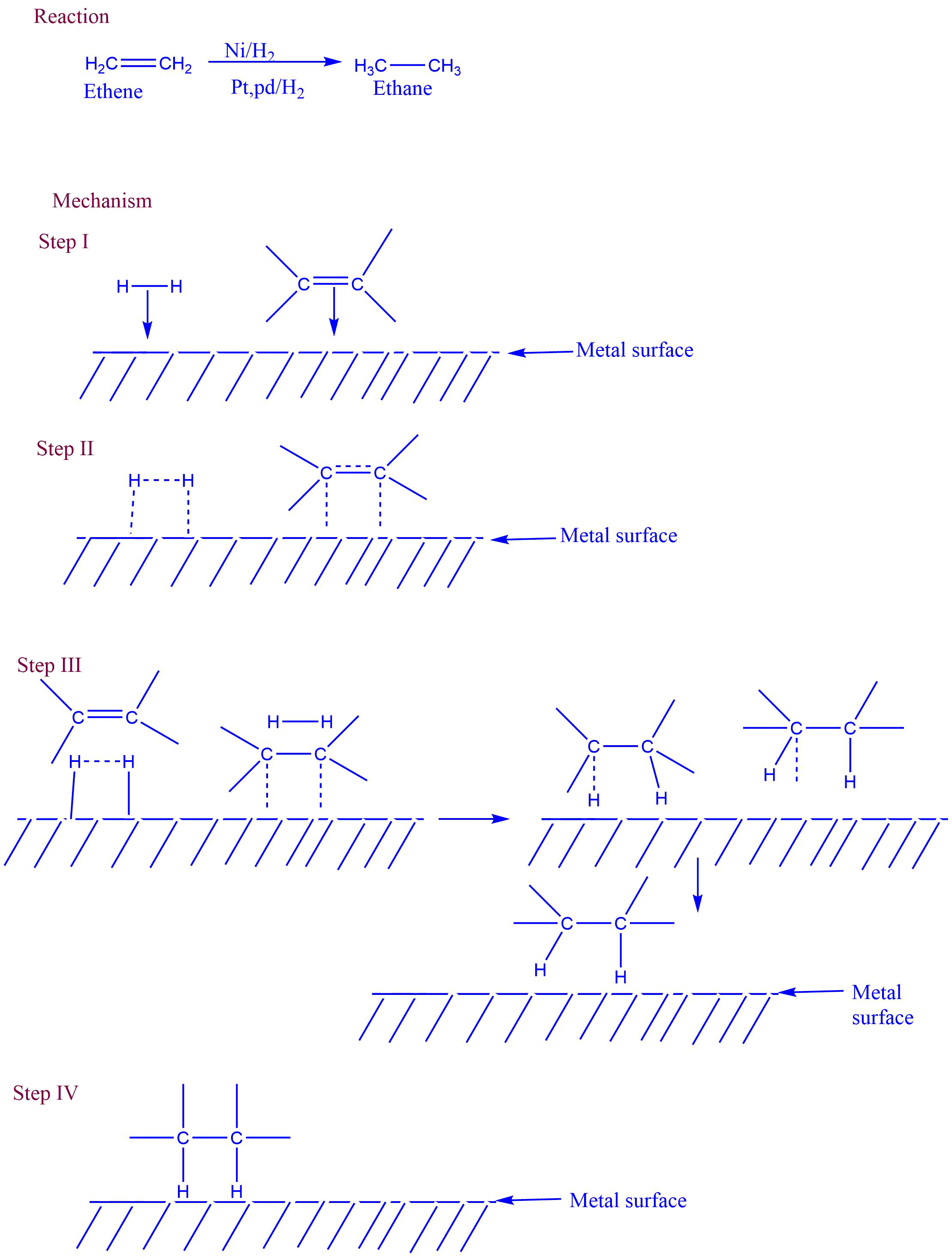
Mechanism of Catalytic hydrogenation
The catalytic hydrogenation of alkane involves different steps they are
I. Diffusion of gaseous reactants
II. Adsorption of reactants to the metal surface
III. Addition of hydrogen to the double bond
IV. Desorption of gaseous product
Applications of catalytic reduction
- It has analytical industrial and preparative applications.
- It determines the number of double and triple bonds present in a compound.
- In the food industry, hydrogenation creates a wide range of products from liquid oils, such as spreads.
Some reactions
- Hydrogenation of cyclohexene

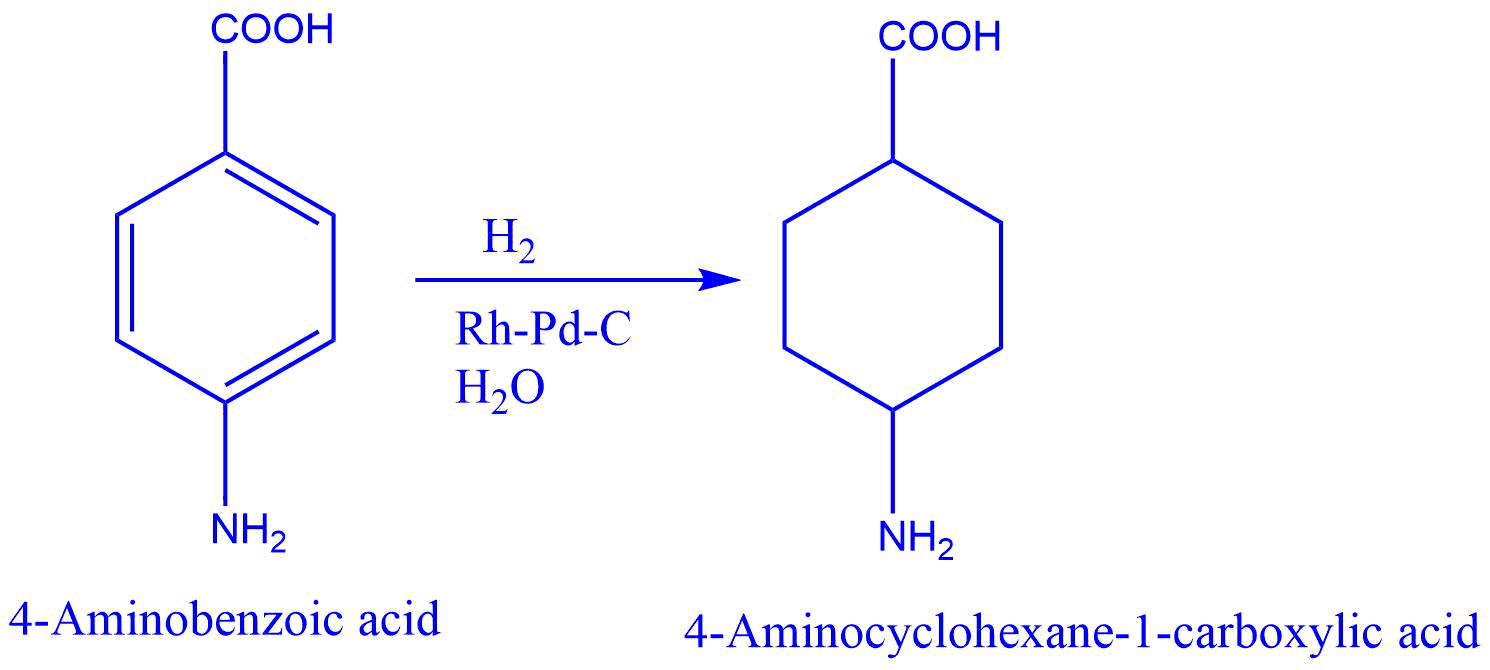
- Reduction of benzoic acid
Metal hydride reduction
Metal hydride reduction is the conversion of aldehyde and ketone into corresponding primary and secondary alcohols in the presence of metal hydrides like lithium aluminium hydride (LiAlH4) or sodium borohydride (NaBH4).
Mechanism of Metal hydride reduction
Step I: Metal hydride provides hydride ion (H–) as an electrophile, which attacks the electron-deficient carbonyl carbon during the nucleophilic addition.
Step II: Lithium aluminium salt of alcohol formed from the first step is hydrolyzed to yield corresponding alcohol.
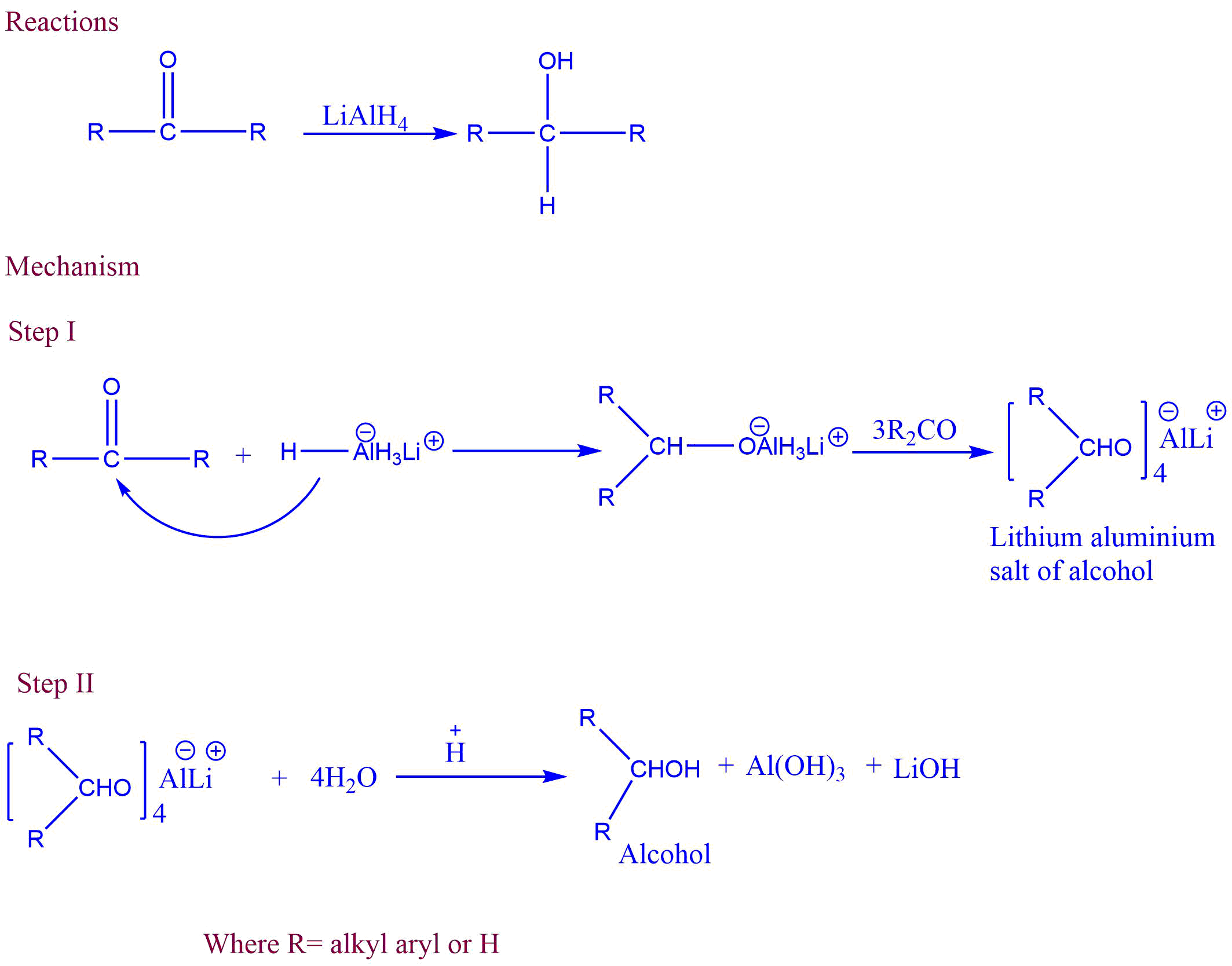
Applications of Metal hydride reduction
- Metal hydride are used to reduced acid hallide, carbocylic acid, ester amide etc.
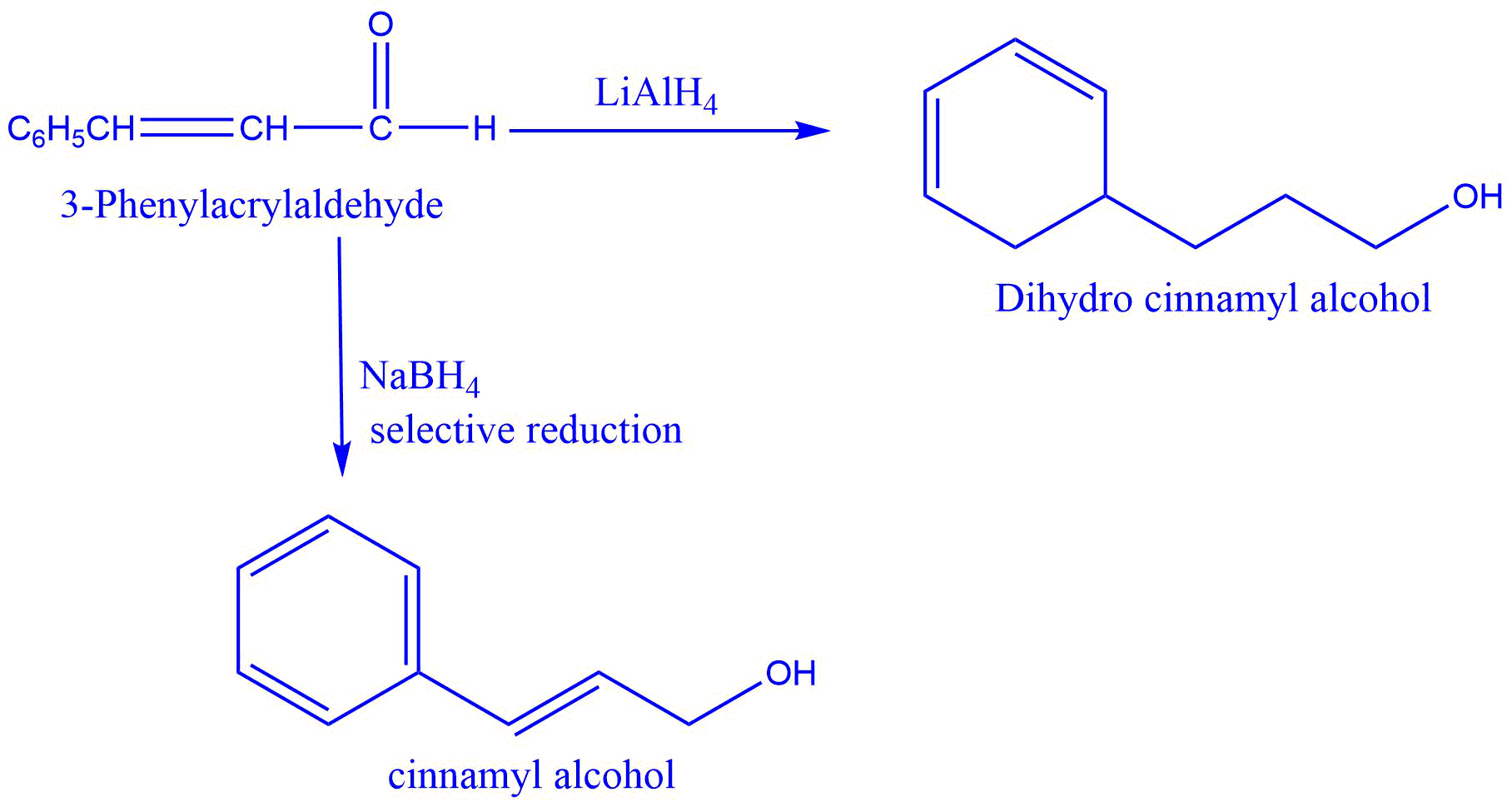
- Sodium borohydride (NaBH4) functions as a selective reducing agent, reducing only carbon-carbon double bonds that is present in conjugation with the -CHO group.
Some reactions
- Reduction of benzoyl chloride benzyl alcohol.
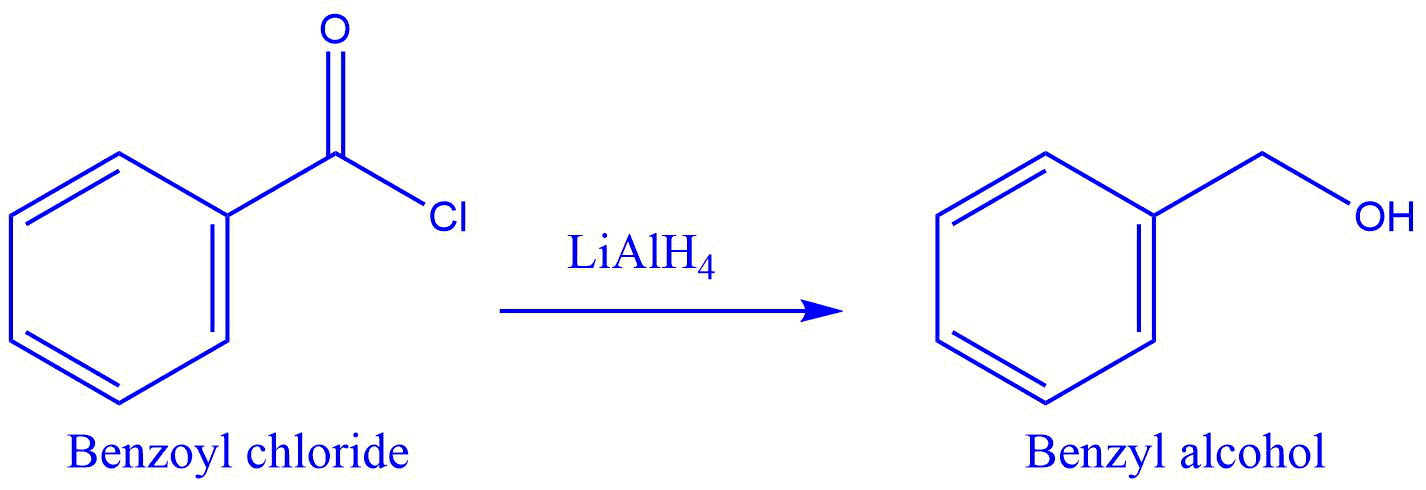
2. Reduction of 3-Phenylacrylaldehyde
Rosenmund reduction
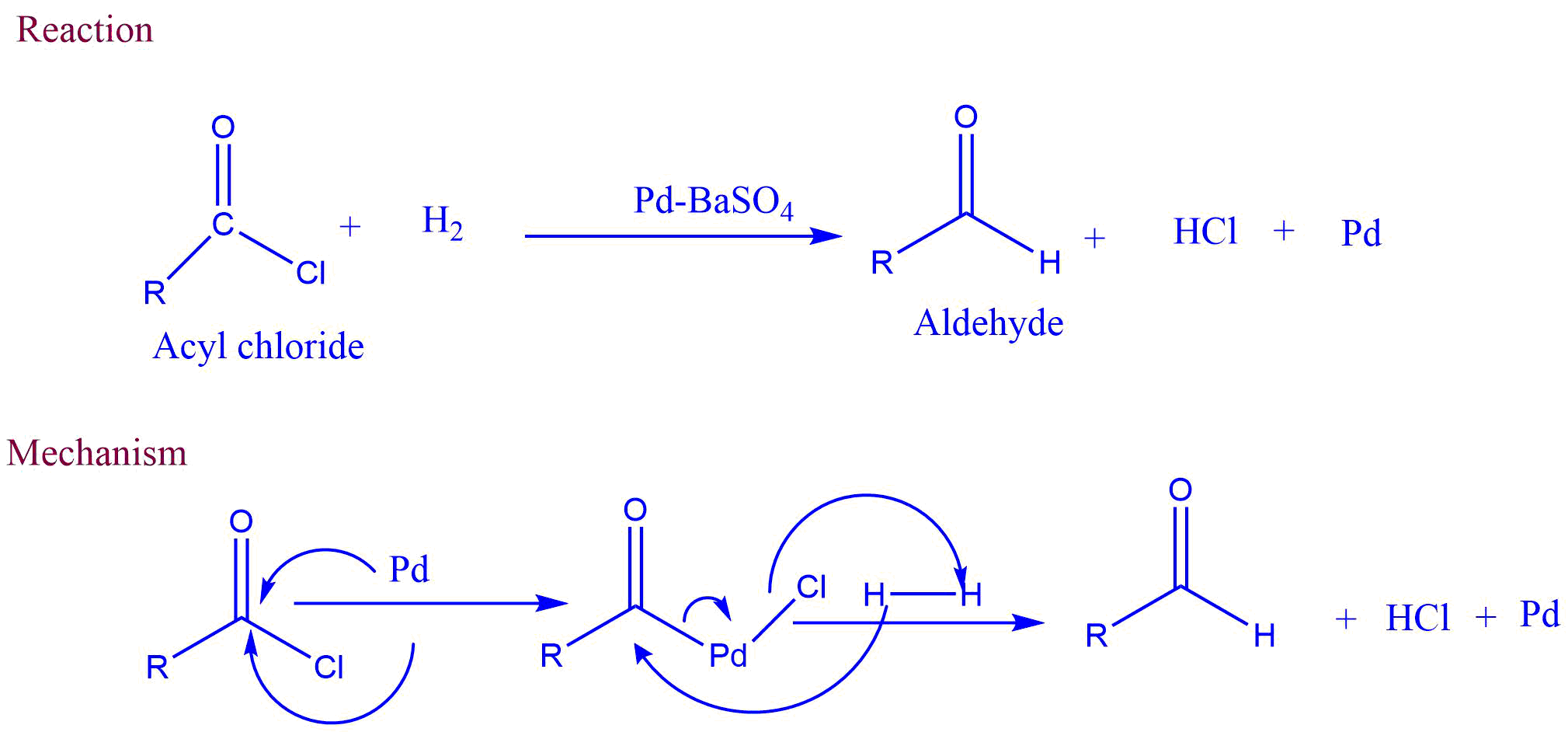
Rosenmund reduction is a reaction that selectively converts acid chlorides to aldehydes by passing hydrogen gas over palladium poisoned by barium sulfate.
Mechanism of Rosenmund reduction
When hydrogen gas is passed through the acyl chloride in the presence of rosenmund catalyst, aldehyde and hydrochloric acid are formed. In this reaction, barium sulfate reduces the activity of palladium and prevents over-reduction.
Video explanation of Rosenmund reduction
Applications of Rosenmund reduction
- It is used for the preparation of aldehyde.
- It is used in the synthesis of saturated fatty aldehydes.
Some reactions
- Conversion of benzoyl chloride to benzaldehyde
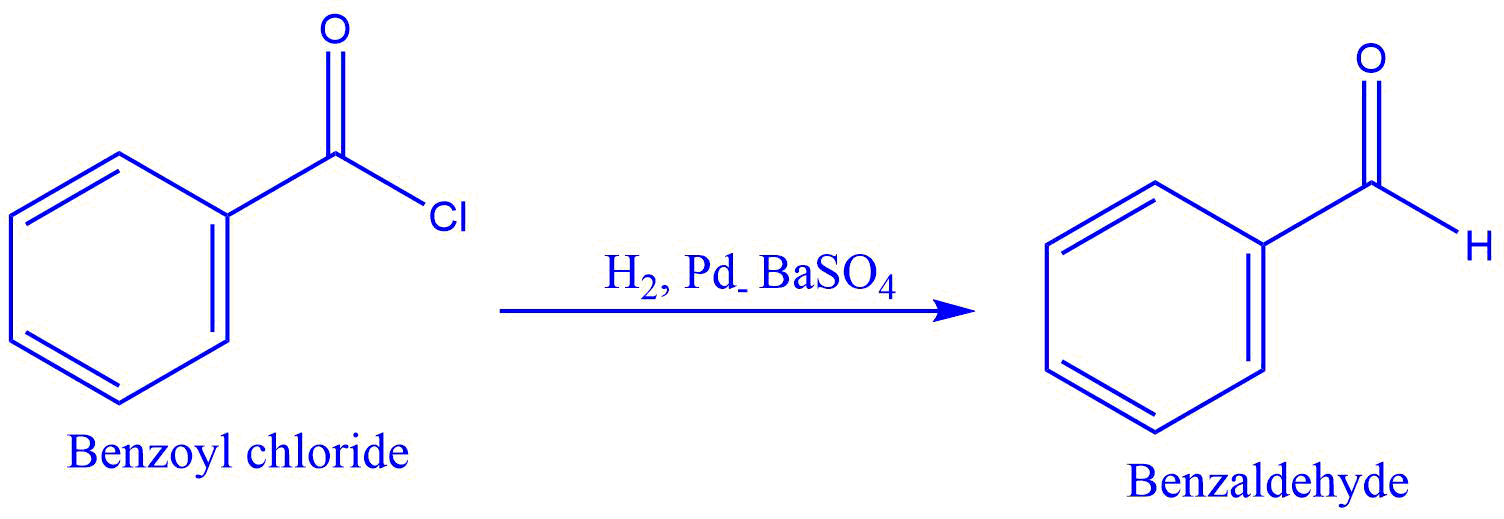
2. Reduction of acetyl chloride to acetaldehyde

References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- https://www.vedantu.com/chemistry/clemmensen-reduction
- https://annamalaiuniversity.ac.in/studport/download/engg/pharm/resources/BPHARM_2Y_4S_401T_Pharm.Org.Chemistry.pdf
- https://byjus.com/chemistry/ronsenmund-reduction-mechanism/
- https://www.organic-chemistry.org/namedreactions/birch-reduction.shtm
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alkenes/Reactivity_of_Alkenes/Catalytic_Hydrogenation
- https://www.pharmaguideline.com/2022/02/metal-hydrid-reduction-nabh4-and-lialh4.html

