Reducing Sugar Definition
Reducing sugar is a type of sugar that consists of a free aldehyde group or a free ketone group, allowing the molecule to act as a reducing agent.
- All monosaccharides are reducing sugars, and so are some disaccharides and oligosaccharides.
- Reducing monosaccharides can further be classified into two groups; aldoses and ketose. Aldoses are sugars consisting of an aldehyde group as the reducing component, whereas ketoses are sugars consisting of a ketone group as the reducing component.
- Ketoses can only reduce other components after they tautomerize into aldoses.
- All disaccharides are not reducing sugars as the aldehyde or ketone group of the molecule might be involved in the cyclic form of the molecule.
- In the case of reducing disaccharides, only one of the two anomeric carbons is involved in the glycosidic bond formation, allowing the other to be free that can convert into an open-chain structure.
- Reducing the property of sugars is important in the case of food as it determines the flavor of the food. The reducing sugar reacts with amino acids in the Maillard reaction when cooked at high temperatures, which are responsible for the flavor of the food.
- The detection of reducing sugars in a sample can be done by one of the two methods; Fehling’s reaction and Benedict’s test.
- The reducing sugar reduces the copper (III) ions in these tests into copper (I) ions resulting in the formation of a brick-red copper oxide precipitate.
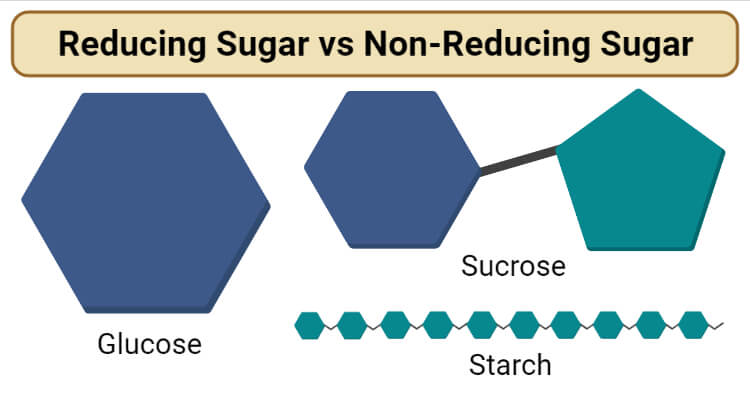
- Some commonly encountered examples of reducing sugars are glucose, fructose, galactose, ribose, etc.
Non-Reducing Sugar Definition
Non-reducing sugar is a type of sugar that doesn’t have a free aldehyde or ketone group, as a result of which the sugar cannot act as a reducing agent.
- All polysaccharides are non-reducing sugars, and so are most disaccharides and oligosaccharides.
- Non-reducing sugars are either dimers, trimers, or polymers, which are formed of many reducing monomeric units by the formation of a glycosidic bond.
- The aldehyde and ketone present on the monomers are involved in the formation of the glycosidic bond in the case of most disaccharides and all polysaccharides.
- One of the most prominent properties of non-reducing sugars is that they do not generate any compounds with an aldehyde group in a basic aqueous solution.
- Non-reducing sugar can be differentiated from reducing sugars through tests like Benedict’s test and Fehling’s test.
- The test is based on the principle of reduction of copper sulfate into copper oxide, which results in the formation of a red brick precipitate.
- In the case of polymeric sugars, the anomeric carbons of all the sugar units are involved in the formation of a glycosidic bond. Thus, these molecules cannot convert into an open-chain form with an aldehyde group.
- Some of the examples of non-reducing sugars include sucrose, trehalose, starch, etc.
9 Major Differences (Reducing Sugar vs Non-Reducing Sugar)
| Characteristics | Reducing Sugar | Non-reducing Sugar |
| Definition | Reducing sugar is a type of sugar that consists of a free aldehyde group or a free ketone group, allowing the molecule to act as a reducing agent. | Non-reducing sugar is a type of sugar that doesn’t have a free aldehyde or ketone group, as a result of which the sugar cannot act as a reducing agent. |
| Reducing nature | Reducing sugar are a good reducing agent. | Non-reducing sugars are poor, reducing agents. |
| Functional groups | Reducing sugars have a free aldehyde or ketone group. | Non-reducing sugar does not have a free aldehyde or ketone group. |
| Fehling’s Test | Reducing sugars give a positive Fehling’s test. | Non-reducing sugars give a negative Fehling’s test. |
| Benedict’s Test | Reducing sugars give a positive Benedict’s test. | Non-reducing sugars give a negative Benedict’s test. |
| Includes | Reducing sugars include all monosaccharides and some disaccharides. | Non-reducing sugars include most disaccharides and all polysaccharides. |
| Taste | Reducing sugars generally have a sweet taste. | Non-reducing sugar generally has a less sweet taste. |
| Molecular weight | Reducing sugars have a lower molecular weight as these are usually of a smaller size. | Non-reducing sugars have a higher molecular weight as they are usually of a larger size. |
| Examples | Some commonly encountered examples of reducing sugars are glucose, fructose, galactose, ribose, etc. | Some of the examples of non-reducing sugars include sucrose, trehalose, starch, etc. |
Examples of Reducing Sugar
Glucose
- Glucose is the most abundant monosaccharide on the plant, which is primarily produced by green algae and plants.
- Glucose is a hexose with six carbon atoms and the molecular formula of C6H12O6.
- It is an aldose consisting of a free aldehyde group at one of the ends, making it a reducing sugar.
- In the solid form, glucose exists in a ring or cyclic form, which converts into an open-chain structure in the aqueous solution.
- The cyclic form of glucose is formed when the hydroxyl group on carbon 5 binds to the aldehyde group on carbon 1.
- Glucose gives a positive Fehling’s, Benedict’s, and Tollen test, which is often used to differentiate glucose from other carbohydrates.
Examples of Non-Reducing Sugar
Starch
- Starch is a polysaccharide composed of multiple monomeric units of glucose linked together by α-1,4 linkages.
- Starch is a non-reducing sugar as it doesn’t have a free aldehyde or ketone group present in the structure.
- The aldehyde or ketone groups on the monosaccharides are involved in the formation of glycosidic bonds that keeps the structure of the molecule.
- Starch gives a negative Tollen’s, Fehling’s, and Benedict’s test as it is a non-reducing sugar.
- Starch is an essential polysaccharide that is used in different industries as well as a source of nutrients in plants. Plants often store starch as a form of glucose storage.
References
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd.
- https://pediaa.com/difference-between-reducing-and-nonreducing-sugar/
- https://vivadifferences.com/difference-between-reducing-sugar-and-non-reducing-sugar-with-examples/

Very helpful 👍👍