Redox reactions can be categorized into two distinct processes, namely oxidation and reduction. The redox reaction, commonly referred to as the Oxidation-Reduction reaction, always includes the simultaneous occurrence of oxidation and reduction phenomena. In the context of a chemical reaction, the species undergoing reduction is commonly referred to as the oxidizing agent, while the species undergoing oxidation is typically referred to as the reducing agent.
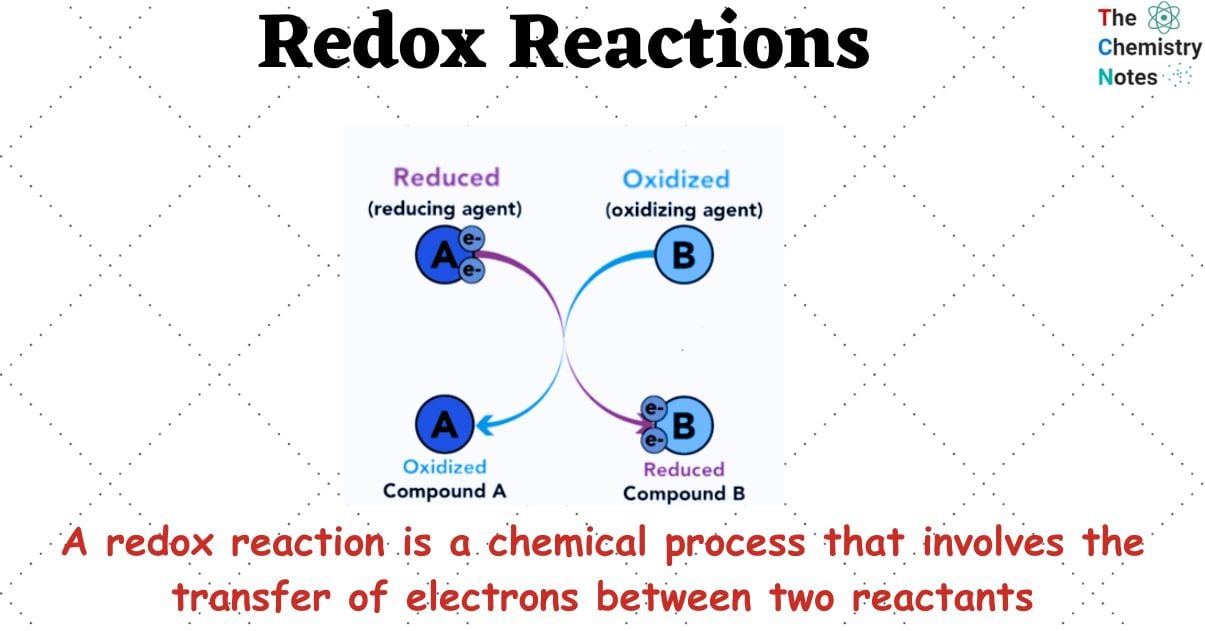
What is Redox Reactions?
A redox reaction is a chemical process that involves the transfer of electrons between two reactants. This electron transfer can be identified by examining changes in the oxidation states of the reacting species.
Redox reactions involve the exchange of electrons between two chemical species. Specifically, one species acts as an electron donor and undergoes oxidation, while the other species acts as an electron acceptor and undergoes reduction.
Oxidation: Oxidation refers to a chemical reaction in which there is a loss of electrons or an increase in the oxidation state of an ion, atom, or specific atoms within a molecule. Commonly, the term “oxidation” refers to a chemical reaction in which an element and oxygen combine.
Reduction: Reduction is a chemical reaction that involves the gain of electrons or a decrease in the oxidation state of an ion, atom, or specific atoms in a molecule.
Example of Redox Reactions
2Cu2O(s) + Cu2S(s) → 6Cu(s) + SO2(g)
- Oxidizing or reducing agent in a chemical reaction, is typically identified by its oxidation state.
- An oxidant is a substance that causes oxidation by accepting electrons, while a reductant is a substance that causes reduction by donating electrons.
- Assigning oxidation numbers to each species involved in a chemical reaction is a crucial step in analyzing the reaction.
- The given chemical equation represents a redox reaction between copper oxide (Cu2O) and copper sulfide (Cu2S), resulting in the formation of copper (Cu) and sulfur dioxide (SO2).
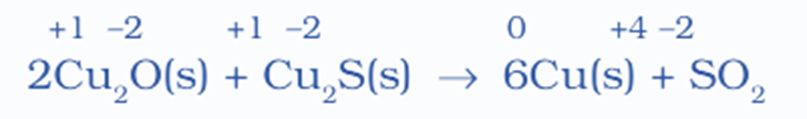
- The coefficients of the balanced equation indicate the stoichiometric ratios of the reactants and products involved in the reaction.
- Based on the evidence presented, it can be inferred that copper undergoes reduction from a +1 oxidation state to a zero oxidation state, while sulfur undergoes oxidation from a -2 oxidation state to a +4 oxidation state during this reaction.
- Cu2O acts as an oxidizing agent for sulfur in Cu2S, leading to an increase in its oxidation number and the formation of Cu(I).
- On the other hand, the sulfur in Cu2S acts as a reducing agent for copper in both Cu2S and Cu2O, causing a decrease in its oxidation number. Therefore, the sulfur in Cu2S can be classified as a reductant.
H2 + F2 → 2HF
The oxidation equation: H2 → 2H+ + 2e–
The reduction equation: F2 + 2e– → 2F–
- The oxidation-reduction reaction between hydrogen and fluorine involves oxidation of hydrogen and reduction of fluorine. The chemical elements hydrogen and fluorine undergo a chemical reaction resulting in the formation of a compound known as hydrogen fluoride.
Types of Redox Reactions
The following are various classifications of redox reactions.
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Disproportionation Reaction
Combination Reaction
The chemical process known as a combination reaction can be represented using the following notation:
A + B → C
For a reaction to be considered a redox reaction, it is necessary that either both A and B are in their elemental form or at least one of them is in the elemental form. It is important to note that any reaction involving the combustion of a substance with elemental dioxygen, as well as reactions that involve elements other than dioxygen, are classified as redox reactions. This category includes several significant examples, such as:
H2 + Cl2 → 2HCl
C + O2 → CO2
Decomposition Reaction
Decomposition reactions are a type of chemical reaction in which a single compound breaks down into two or more simpler substances, which can be elements or compounds. They are the reverse of combination reactions, which involve the combination of two or more substances to form a single compound.
2 H2O2 → 2 H2O + O2
Na2CO3 → Na2O + CO2
Decomposition reactions refer to a type of chemical reaction where a single compound breaks down into two or more simpler substances. There are three main types of decomposition reactions:
- Thermal decomposition,
- Electrolytic decomposition, and
- Photolytic decomposition.
Thermolysis: a chemical reaction that involves the breakdown of a substance due to the application of heat.
Electrolysis: decomposition of matter caused by electricity.
Photolysis: chemical process in which a substance is broken down into smaller components as a result of exposure to light.
Displacement Reactions
Displacement reactions refer to chemical reactions in which an atom or ion in a compound is replaced by another atom or ion. A displacement reaction involves the substitution of an ion or atom within a compound with an ion or atom of a different element.
The chemical reaction can be represented as follows:
X + YZ → XZ + Y
Displacement reactions can be classified into two distinct categories. The phenomenon of metal displacement and non-metal displacement is a topic of interest in the field of chemistry.
Displacement reactions refer to chemical reactions in which an element or group of elements in a compound is replaced by another element or group of elements.
Single displacement reaction, also referred to as a single replacement reaction, is a form of oxidation-reduction chemical reaction. In this reaction, an ion or element is displaced from a molecule, resulting in the replacement of one element in a compound with another.
Double displacement reactions involve the transfer of a portion of two ionic compounds, leading to the creation of two novel components. The aforementioned is indicative of a double displacement reaction. Double displacement processes occur in aqueous solutions as ions undergo precipitation and exchange of ions.
Displacement reactions can be classified into two distinct categories, namely metal displacement and non-metal displacement.
Metal Displacement: A metal is typically replaced by another metal in metal displacement reactions. In the metallurgical process, metal displacement processes are employed to separate pure metals from their ores.
CuSO4+Zn → Cu+ZnSO4
Non-Metal Displacement: Hydrogen H2 or occasionally oxygen O2 is utilized to replace metal in non-metal displacement reactions.
Disproportionation Reactions
Disproportionation reactions refer to chemical reactions wherein one single reactant undergoes both oxidation and reduction. One real-world example of such a process is the reaction that occurs when hydrogen peroxide is applied to a wound. Since hydrogen peroxide breaks down to create oxygen and water, this may initially appear to be a straightforward breakdown reaction.
The other example of disproportion reaction is:
P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
Redox Reaction Examples in Daily Life
- Cellular respiration, which serves as the primary source of energy in human organisms, involves a sequence of oxidation-reduction reactions. The process by which the energy from the food we ingest is converted is exclusively through redox reactions.

- Combustion is a real-life example of a redox reaction. The process of burning organic material and fossil fuels involves redox reactions. During combustion, the carbon and hydrogen in the compound react with oxygen from the atmosphere to form new compounds. Combustion is a chemical reaction where a substance is burned in the presence of oxygen from the atmosphere. During this process, the oxygen is consumed while the substance being burned undergoes oxidation.
- Corrosion is a common occurrence that involves redox reactions. When water comes into contact with metal, such as an iron door, some of the oxygen atoms in the water react with the metal and cause the production of hydrogen ions. This process is called oxidation. Hydrogen ions combine with oxygen to produce water, and this process repeats itself.
- Photosynthesis occurs in the leaves of plants. During the process of photosynthesis, carbon dioxide and water react with sunlight to produce oxygen and glucose. During photosynthesis, glucose is produced and used by plants to fuel their metabolic reactions. During photosynthesis, the process of oxidation occurs in water, while the process of reduction occurs in carbon dioxide.
- Decay or decomposition is an example of redox reactions. Living matter in nature is composed mainly of carbon, hydrogen, and oxygen. When an organism dies, the organic compounds within it begin to react with oxygen. The reaction mentioned earlier is a lengthy process. The process of decay or decomposition is an example of redox reactions.
Significance of Redox Reactions
- Oxidation-reduction reactions are important because they are the main source of energy on Earth, both in natural (biological) and non-natural (artificial) processes. In an oxidation reaction, a significant amount of energy can be obtained through either the elimination of hydrogen or the fusion of oxygen.
- The oxidation process is utilized in the production of industrial cleaning products.
- Galvanization is a process that involves coating steel with a layer of zinc.
- Nitric acid is produced through the oxidation of ammonia, and it is an important fertilizer.
- Redox reactions are utilized in the process of extracting metals from their ores. One example of this is the smelting of metal sulfide with a reducing agent present.
- A redox reaction is utilized in the production of gold-plated ornaments to deposit a thin layer of material onto the object’s surface. The process is referred to is commonly known as electroplating.
- Cellular respiration is a process that involves the oxidation of glucose (C6H12O6) and the reduction of oxygen (O2) to produce carbon dioxide (CO
2) and water (H2O). Cellular respiration involves the process of redox, which includes the conversion of NAD+ to NADH through reduction and the conversion of NADH to NAD+ through oxidation. - Redox reactions have various applications, such as: Mineral mobilization, Formation of minerals, Depositional environments.
- Minerals are formed through various geological processes such as crystallization from magma or lava, precipitation from water or mineral-rich fluids, and metamorphism caused by heat and pressure. These processes can occur over millions of years and result in a wide range of mineral types with unique physical and chemical properties.
Frequently Asked Questions (FAQ)
What are redox reactions?
Redox reactions are chemical reactions that involve the transfer of electrons between two species. One species loses electrons (oxidation) while the other gains electrons (reduction).
Is there double replacement in acid-base reactions?
Double displacement reactions involve the exchange of anions or cations between two ionic compounds. Neutralization reactions happen when an acid and a base react. These reactions are usually favorable if the reaction involves a solid acid and/or base.
To determine whether a redox reaction is occurring in an acidic, alkaline, or neutral medium, you can use indicators or pH measurements.
How would you determine whether a redox reaction is taking place in an acidic/alkaline or neutral medium?
When H+ or any acid is on both sides of a chemical equation, the processes take place in an acidic medium. If OH– or any other base shows up on both sides of a chemical equation, the solution is said to be basic. The solution is neutral if there are no H+, OH–, acids, or bases in the chemical equation.
Video on Redox Reaction
References
- Schüring, J.; Schulz, H. D.; Fischer, W. R.; Böttcher, J.; Duijnisveld, W. H., eds. (1999). Redox: Fundamentals, Processes and Applications. Heidelberg: Springer-Verlag ISBN 978-3-540-66528-1.
- Bockris, John O’M.; Reddy, Amulya K. N. (1970). Modern Electrochemistry. Plenum Press.
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2017). General Chemistry: Principles and Modern applications
- https://studiousguy.com/examples-redox-reactions-everyday-life/
- https://www.biologyonline.com/dictionary/redox-reaction
- https://byjus.com/jee/redox-reactions/
- https://www.geeksforgeeks.org/redox-reactions-definition-types-uses-applications/
- https://www.bbc.co.uk/bitesize/guides/z2r44wx/revision/3
- https://studymind.co.uk/notes/redox-processes/
- https://blog.praxilabs.com/2022/05/17/20-oxidation-reduction-examples/

