The relative amounts of reactants and products present during a reaction at a specific time are determined by the reaction quotient (Q). The reaction quotient assists in determining which direction a reaction is likely to go given the pressures or concentrations of the reactants and products.
The equilibrium constant, Keq, provides us with the product-to-reactant ratio at equilibrium, whereas the reaction quotient, Q, gives us the product-to-reactant ratio at any point in time.
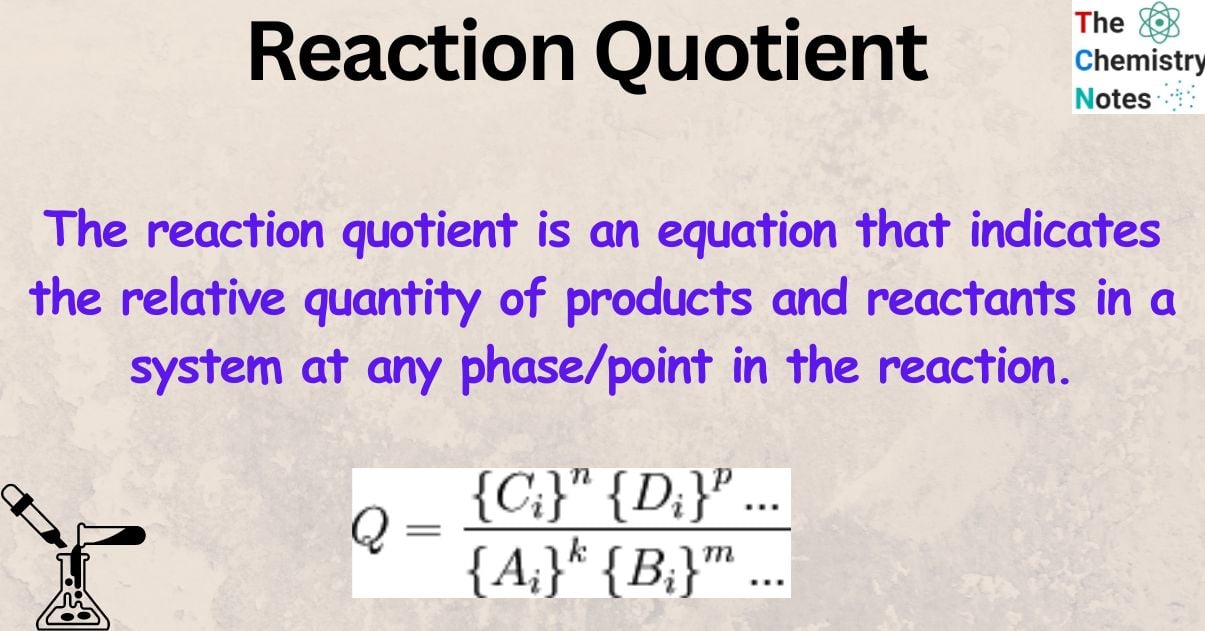
What is Reaction Quotient?
The reaction quotient is an equation that indicates the relative quantity of products and reactants in a system at any phase/point in the reaction.
A reaction quotient, Q, is utilized to determine the direction of the reaction.
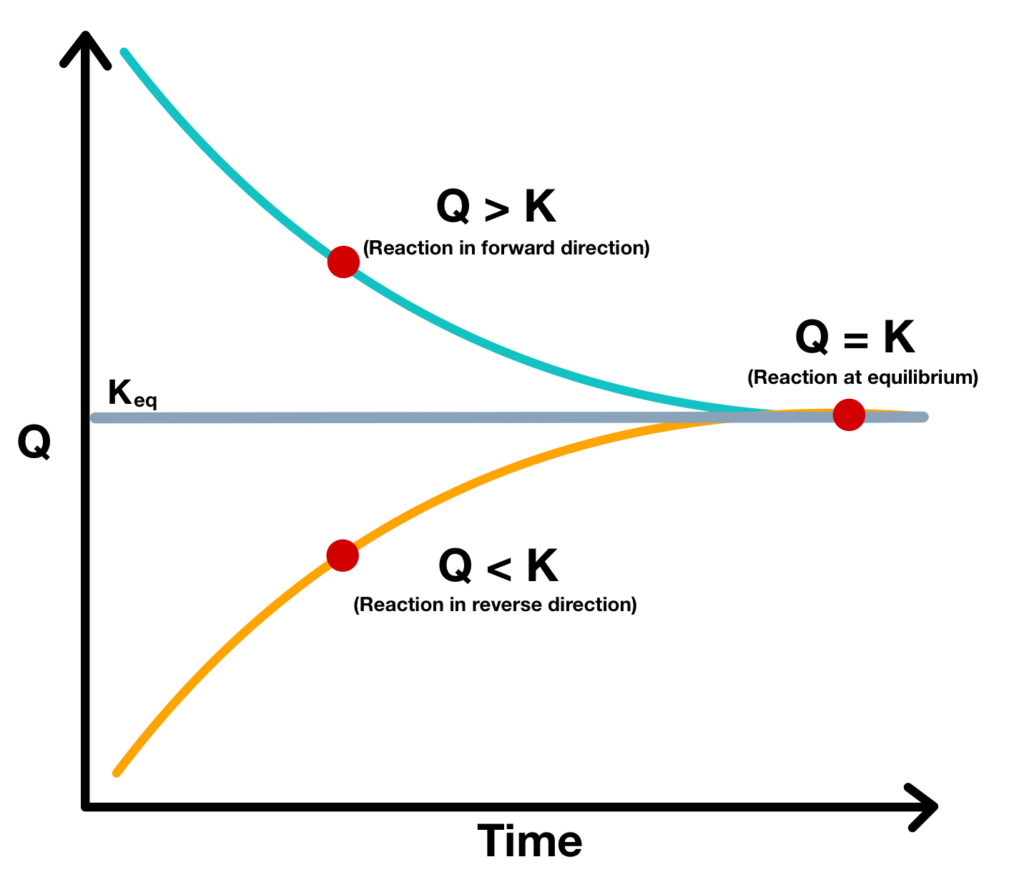
When the reaction quotient is compared to the reaction rate, K, it can indicate if the reaction is moving forward or backward, or if it is at equilibrium.
- When Q < K, the reaction is in the reverse direction
- When Q > K, the reaction is in the forward direction
- When Q = K, the reaction is at equilibrium
In chemical thermodynamics, the reaction quotient (Q) is a number that offers a measurement of the relative amounts of products and reactants present in a reaction mixture at a given moment in time for a reaction with well-defined overall stoichiometry. It is defined theoretically as the ratio of the product species’ activity (or molar concentrations) to those of the reactant species engaged in the chemical reaction, with the stoichiometric coefficients of the reaction taken into consideration as exponents of the concentrations. When the system is in equilibrium, the reaction quotient is constant over time and equal to the equilibrium constant.
Reaction Quotient Expression
Given a general equilibrium expression;
A + mB …  nC + pD …
nC + pD …
where;
A, B, C, and D are chemical species involved in this reaction and
k, m, n, and p are the stoichiometric coefficients for the reaction,
The reaction quotient, Q, is defined as
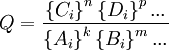
where the {Ai} denotes the instantaneous activity of the species A at a certain moment of time and so on for the other species.
The reaction quotient is calculated at a specific point in time, not necessarily when equilibrium is reached. Le Chatelier’s Principle is closely related to the reaction quotient.
Types of Reaction Quotient
We can obtain several forms of reaction quotients. Among various types, two of the reaction quotient types are:
Qc is comparable to Kc: It determines the concentration of aqueous or gaseous species in a system at a certain time.
Qp is comparable to Kp: It quantifies the partial pressure of gaseous species in a system at a specific point in time.
Calculating Reaction Quotient
When the reaction is not in equilibrium, the reaction quotient is expressed in terms of molar concentration as,

- The concentration of a species at a specific time is shown in square brackets. As a result, [A] denotes the concentration of species A.
- The superscript lowercase letters are exponents calculated from the species coefficients in the balanced chemical equation. As a result, [A]a denotes the concentration of species A multiplied by the number of moles of A in the balanced equation.
- Overall, the numerator represents the product concentrations raised to the power of their coefficients and multiplied together.
- The denominator indicates the concentrations of the reactants multiplied by the power of their coefficients. Simply divide the numerator by the denominator to find Qc.
And the reaction quotient in terms of the components’ partial pressures as,

- P denotes a species’ partial pressure at a specific time. As a result, (PA) denotes the partial pressure of species A.
- The superscript lowercase letters are exponents calculated from the species coefficients in the balanced chemical equation. As a result, (PA)a denotes the partial pressure of species A multiplied by the number of moles of A in the balanced equation.
- Overall, the numerator indicates the partial pressures of the products multiplied by the power of their coefficients.
- The denominator indicates the reactants’ partial pressures increased to the power of their coefficients and then multiplied together. Simply divide the numerator by the denominator to find Qp.
Example of a Reaction Quotient
We must first create an expression for Qc in order to calculate it for the given reaction. The reversible reaction has the following equation:
N2(g) + 3H2(g) ⇌ 2NH3(g)
- We can now write an expression for Qc using the reactant and product concentrations at a specific time.
- We discover the concentrations of the products as the numerator, which are all increased to the power of their coefficient in the chemical equation and then multiplied together.
- The only product in this equation is NH3, and there are two moles of it. As a result, the numerator is [NH3]2.
- We discover the concentrations of the reactants as the denominator, which are all increased to the power of their coefficient in the chemical equation and then multiplied together. The reactants in this case are N2 and H2. There is one mole of N2 and three moles of H2. As a result, our denominator is [N2] [H2]3
When we put all of this together, we get the following expression for Qc:
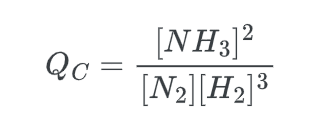
All that remains is to substitute the concentrations as per the questions asked.
Thus, the resulting value can be compared to the reaction’s equilibrium constant, Kc, to determine whether the reaction is at equilibrium and, if not, the path the reaction will take to achieve equilibrium. If Qc is less than Kc, the reaction will continue until it reaches equilibrium. If Qc exceeds Kc, the reaction will proceed in the opposite direction to attain equilibrium. When Qc equals Kc, the reaction is said to be in equilibrium.
Unit of Reaction Quotient
Reaction Quotient is unitless.
Q is conceptually focused on activities, much like Keq. The concentration of a substance at any given time during a reaction is actually its concentration activity, which is its concentration in relation to the species’ typical concentration. Since both quantities are commonly expressed in M (or mol dm-3), the units cancel out and leave a unitless value. Similar to partial pressure, we measure pressure activity, which is the substance’s partial pressure in relation to a reference pressure. Once more, there are no units for pressure activity. Q is unitless because both of its forms are composed of unitless values.
References
- https://www.expii.com/t/reaction-quotient-definition-overview-8522
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/reaction-quotient/
- https://shiken.ai/chemistry/reaction-quotient
- Zumdahl, Steven; Zumdahl, Susan (2003). Chemistry 6th Edition. Houghton Mifflin Company. ISBN 0-618-22158-1.
- https://infinitylearn.com/surge/blog/iit-jee/reaction-quotient/
