A reaction mechanism refers to the series of elementary steps that take place during a chemical reaction. A reaction that takes place in two or more elementary steps is referred to as a multistep or complex reaction. A reaction intermediate refers to a chemical species that is produced in one elementary step and then utilized in a subsequent step. The step in a reaction mechanism that proceeds at the slowest rate is referred to as the rate-determining step.
The rate-determining step is responsible for limiting the overall rate of the reaction and, as a result, it also determines the rate law for the entire reaction.
Interesting Science Videos
Elementary reactions
Elementary reactions are one-step processes with a constant order and molecularity.
In other words, the expressions for rate law and the law of mass action are the same.
The term “molecularity” refers to the total number of molecules that are involved in the effective collision of an elementary step. The concept of molecularity can be utilized to categorize elementary steps into three distinct groups:
- Unimolecular reactions involve the participation of only one molecule.
- Bimolecular reactions involve the participation of two molecules.
- Termolecular reactions involve the participation of three molecules.
There are only three primary categories of molecularity because it is uncommon for more than three molecules to participate in an effective collision simultaneously.
Complex Reactions
- These multi-step reactions may or may not have the same order and molecularity.
- In these kinds of reactions, intermediates are created throughout the process that are distinct from the reactants and the finished products.
- This reaction’s individual steps are all elementary reactions.
- he reaction’s overall speed will match that of the slowest step. As a result, the slowest step in a reaction is referred to as the rate-determining step (RDS).
- The order of each reaction may differ from the overall order of the reaction. The concentrations included in the rate law expression of RDS determine this. If it is not in overall order, it can be calculated using the steady-state approximation or the equilibrium approach.
- Order and molecularity are not the same for complex reactions.
Reaction Mechanism
Mechanism refers to the steps in a reaction and the process of identifying the slowest or rate-determining step.
The reaction mechanism is a set of actions (sometimes referred to as elementary processes) that result in the formation of products or are responsible for the overall chemical reaction.
For Example: The reaction between H2 and I2 to form hydrogen iodide was originally postulated as a simple one-step reaction.
H2 + I2 = 2HI
Rate = k[H2][I2]
Reaction mechanism of the formation of HI
I2 → 2I (fast) ………i)
H2 + I → H2I (fast)……..ii)
H2I + I → 2HI (slow)……..iii)
Overall reaction: H2+ I2 → 2HI
Multistep Reaction Mechanism
Multistep reaction mechanisms refer to chemical reactions that occur in multiple steps, with each step involving a different chemical reaction. These mechanisms are important in understanding the overall reaction and can provide insight into the intermediates and transition states involved.
Chemists can start exploring potential reaction mechanisms once they have determined the experimental rate law for a reaction. To be considered a possible reaction mechanism, it must satisfy at least two conditions:
- The equations representing the individual elementary steps in the mechanism should sum up to the overall equation of the reaction.
- The mechanism should be in accordance with the experimental rate law.
For Example: Reaction between NO2 and CO are generally believed that this reaction occurs through two elementary steps:
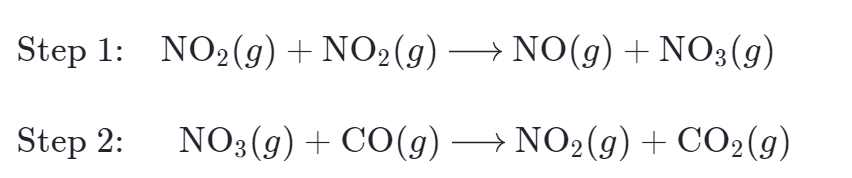
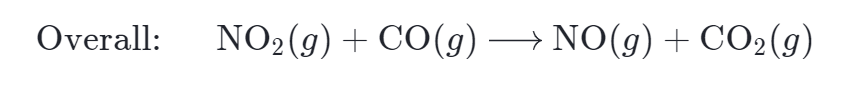
The species NO3 is present in both steps of the mechanism, but it does not appear in the overall equation. In this case, the reaction intermediate is a species that is formed in one step and consumed in a subsequent step.
Now, we need to verify if the experimental rate law is in agreement with the two-step mechanism. In order to proceed, it is necessary to determine which of the two steps is the rate-determining step, or the slowest step in the mechanism. The rate-determining step plays a crucial role in determining the overall rate of a reaction since a reaction cannot occur faster than its slowest step.
In our proposed mechanism, the rate-determining step is believed to be step 1:
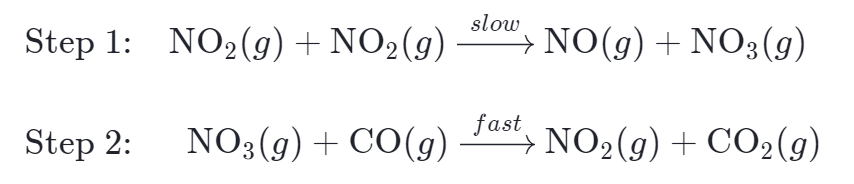
The rate law for step 1 will be equivalent to the overall rate law, as it limits the overall rate of the reaction. Therefore, the predicted rate law for the overall reaction is:
rate=k[NO2]2
The rate law agrees with the previously determined experimental rate law, indicating that the mechanism satisfies the second condition. As the reaction mechanism satisfies both conditions, we can confidently conclude that it is a valid reaction mechanism.
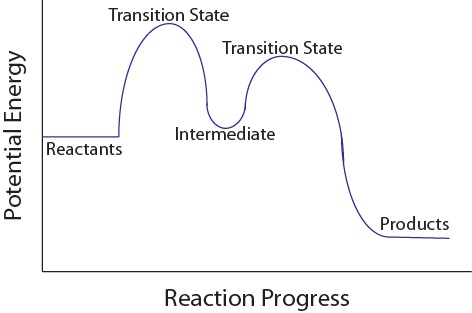
Rate Determining Step in Multistep reaction mechanism
In multi-step mechanisms, it is common for one step to be considerably slower than the others. The step that proceeds at the slowest rate is commonly known as the rate-determining step, since it restricts the overall reaction rate.
One analogy that can help illustrate this concept is that of an hourglass with two openings of different sizes. The smaller of the two openings in the hourglass determines the rate at which the sand falls into the bottom-most chamber. The rate law for the overall reaction can be determined by identifying the slowest step, which is also known as the rate-determining step.
Identify the rate-determining step and write the overall reaction for the multi-step reaction provided below.
H2O2 (aq) + I− (aq) → IO− (aq) + H2O (l) (fast)
H2O2 (aq) + IO− (aq) → I− (aq) + H2O (l) + O2 (g) (slow)
Overall reaction: 2 H2O2 (aq) → 2 H2O (l) + O2 (g)
To determine the overall reaction, the two elementary steps are combined and any identical species that appear on both sides of the chemical equation, such as I− (aq) and IO− (aq), are cancelled out.
References
- https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:kinetics/x2eef969c74e0d802:reaction-mechanisms/a/reaction-mechanisms
- https://opentextbc.ca/introductorychemistry/chapter/reaction-mechanisms/
- https://boisestate.pressbooks.pub/chemistry/chapter/17-6-reaction-mechanisms/
- https://openstax.org/books/chemistry-2e/pages/12-6-reaction-mechanisms
