Several substances, including uranium and radium, are unstable. Their atomic nucleus spontaneously disintegrates into a smaller atomic nucleus of a different element. To create the new nucleus, the protons, and neutrons in the unstable nucleus recombine. Radiation, which we refer to as a discharge of extra particles and energy from the initial nucleus, results from this. Radioactive elements are those whose atomic nuclei produce radiation. Radioactive disintegration or radioactive decay is the term used to describe the spontaneous breakdown of unstable atoms.
The disintegration or decay of unstable atoms accompanied by the emission of radiation is called radioactivity.
Interesting Science Videos
What is Radioactivity?
Atomic nuclei that are unstable will spontaneously split apart to generate nuclei that are more stable. This process of decomposition is called radioactivity. The energy and particles released during the breakdown process are referred to as radiation. These are, in other words, the particles that the nuclei emit as a result of nuclear instability.
The process through which unstable nuclei disintegrate in nature is known as natural radioactivity. When we cause the breakdown of unstable nuclei in a lab, we create artificial radioactivity. Radioactive decay is a characteristic of several naturally occurring elements and synthetic isotopes.
Types of Radioactivity/Radiation
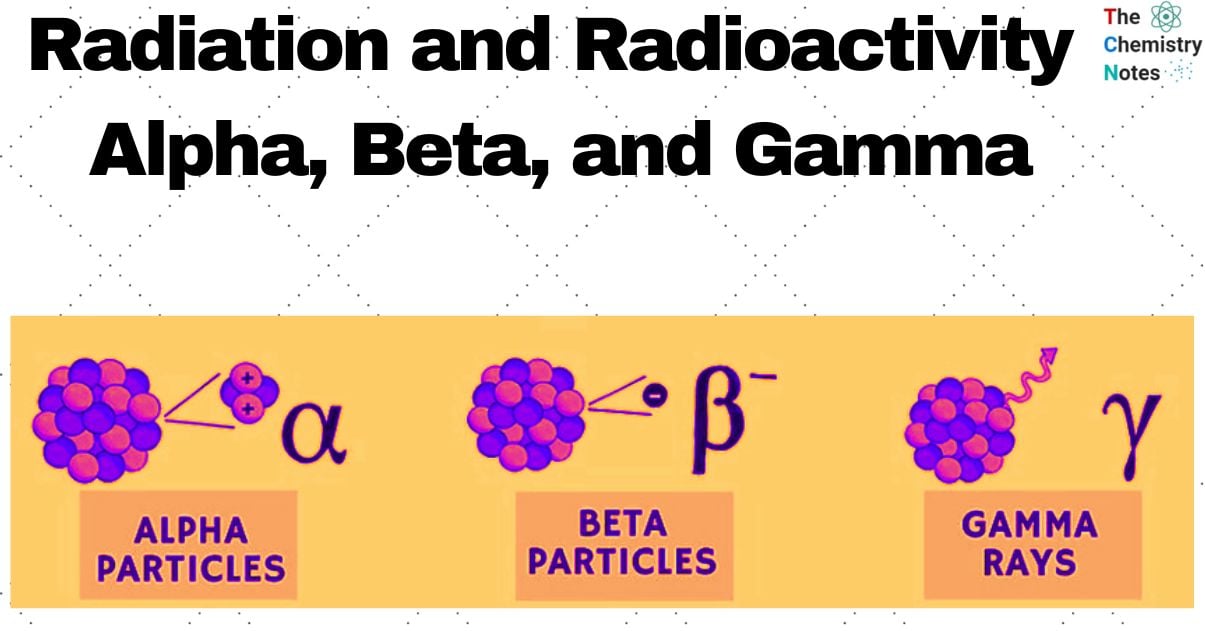
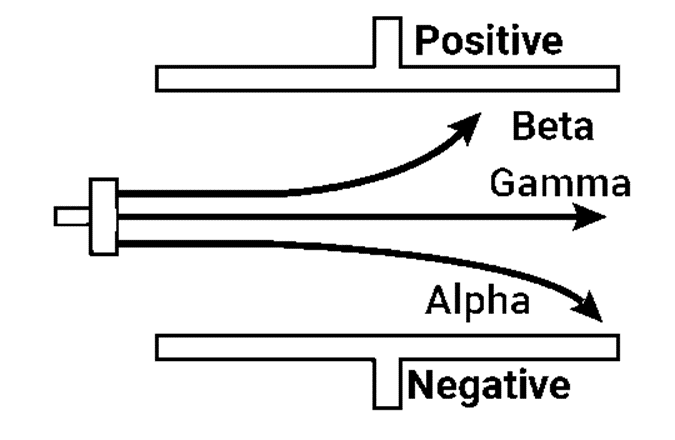
There are three different types of radioactive radiation. Rutherford (1902) segregated these by passing them between two plates with opposing charges. The positive charge, or “alpha” rays, were those that bent toward the negative plate. The negative charge-carrying objects that bent in the direction of the positive plate were known as (beta) rays. The third form of radiation, known as (gamma) rays, was uncharged and traveled directly through the electric field. As they induce luminescence on the zinc sulphide screen put in their path α, β and γ rays could be easily seen.
Properties of Radiations
The three types of radiations; Alpha (α), beta (β) and gamma (γ) rays differ in nature and properties as described below:
Alpha (α) rays
- Nature: Rutherford demonstrated their e/m ratio and thus their 4 amu mass and +2 charge. These are nuclei of helium, and their symbols are 2α4 or 2He4.
- Velocity: Radioactive nuclei emit α-particles at a very high velocity—roughly one-tenth the speed of light.
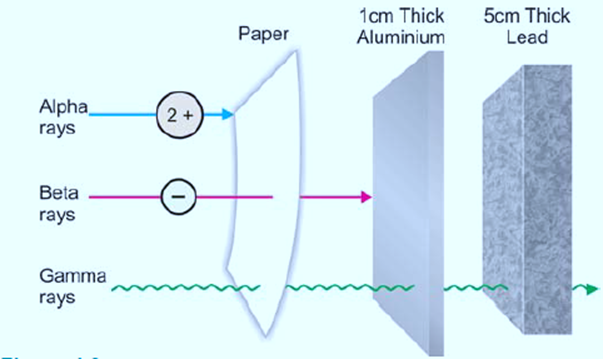
- Penetrating power: The power of penetration of α-particles through matter is extremely low due to their charge and size.
- Ionisation: They induce a gas they pass through to ionize intensely. Due to their rapid speed and attraction to electrons, α-particles displace electrons from gas molecules and change them into positive ions.
Beta (β) rays
- Nature: These are streams of beta-particles that the nucleus emits. Becquerel demonstrated that β-particles are identical to electrons by observing their deflection electric and magnetic forces. They have an extremely low mass of 1/1827 amu and a charge of -1. The symbol for β -particle is -1β0 or -1e0.
- Velocity: They travel about 10 times faster than α-particles. Their velocity is about the same as of light.
- Penetrating power: Compared to α-particles, β-particles are 100 times more penetrating. This is the case because of their low mass and higher velocity. A layer of aluminum that is 1 cm thick or 1 m of air can stop β-particles.
- Ionisation: Around one hundredth of what is created by -particles in a gas in terms of ionization. The kinetic energy of β-particles is substantially lower than that of α-particles, despite the fact that their velocity is higher and their mass is smaller. They, therefore, make poor ionizers.
Gamma (γ) rays
- Nature: They don’t contain matter particles like α and β-rays do. X -rays are a type of electromagnetic radiation, whileγ -rays have a shorter wavelength. These could be described as nuclear emissions of high-energy photons during α- or β-emissions. They can be represented as 0γ0.
- Velocity: Like all forms of electromagnetic radiation, γ-rays travel with the velocity of light.
- Penetrating power: γ-rays are the most penetrating because of their high velocity and non-material nature. Even a coating of lead that is 5 cm thick or a slab of concrete that is several meters thick cannot stop them.
- Ionisation: Compared to α- and β-particles, their ionizing power is quite low. A positive ion is produced when a γ-photon knocks out an electron from a gas molecule. As a result of the low probability of photon-electron collisions,γ -rays are weak ionisers.
Difference Between Alpha, Beta, and Gamma Rays
| S.N. | Properties | α- rays (or particles) | β- rays (or particles) | γ- rays |
| 1. | Representation | It is represented as 24He or He++. | It is represented as -10e. | It is represented as 00 γ. |
| 2. | The action of the electric field | They deviate towards the cathode. | They deviate towards the anode. | They do not deviate. |
| 3. | Ionizing power | They have high ionizing power, nearly 100 times to that of β- rays. | They have high ionizing power, nearly 100 times that of γ – rays | They have lower ionizing power than α and β-particles. Penetration power |
| 4. | Penetration power | They have little penetration power through solid substances and get scattered by thin foils of metals. They can penetrate a few centimeters of air. | They can penetrate 1-2 m in the air. | They have a greater penetration power than α and β-particles and can penetrate through several centimeters of iron and lead sheets. |
| 5. | Nature of product | The product obtained by the loss of α – particles has an atomic number less by two units and a mass number less by four units than that of the parent element. | The product obtained by the loss of β- particles have an atomic number of more than one unit without any change in mass number. | There is no change in the atomic number as well as in the mass number. |
Determination of Radioactivity
The radioactive radiation can be detected and measured by a number of methods. The important ones used in modern practice are listed below.
Cloud chamber
Detecting radioactivity makes use of the method depicted in Fig below. There is a lot of water vapor in the air inside the chamber. The gas expands and is supercooled when the piston is rapidly lowered. Ions are produced anywhere α- or β-particle travels through a gas.
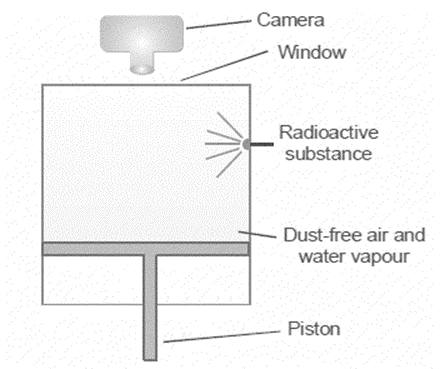
Condensed water droplets form on the surfaces of these ions. The resulting cloud or trail can be used to trace the path of the particle. The window above allows for instantaneous photographs of the track below. The passage of α- or β-particles through liquid hydrogen also leaves a trail of bubbles. The particle tracks can be better photographed using the bubble chamber method.
Ionisation Chamber
This is the simplest device used to measure the strength of radiation. In an ionization chamber, air flows between two metal surfaces. This chamber is used to create positive ions by removing electrons from gas molecules by radiation. The anode receives the electrons while the cathode receives the positive ions.
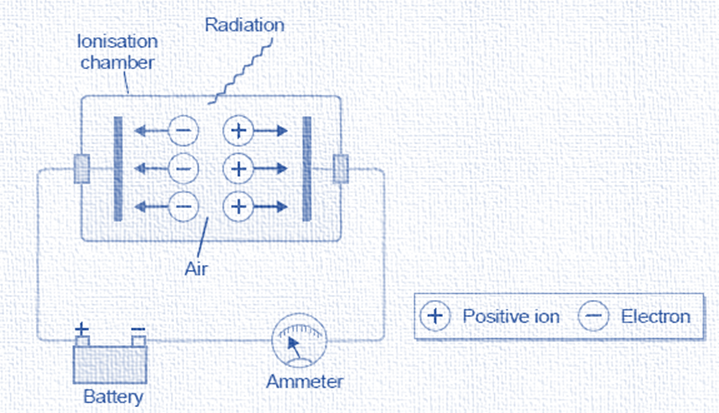
As a result, a minimal electric current flows between the plates. The strength of the radiation that enters the ionization chamber can be determined by measuring the current flowing through it, which can be done with an ammeter. The quantity of electric charge that flows between two plates in a particular amount of time is measured in an ionization chamber called a dosimeter. The amount of radiation that has passed through the chamber is directly proportional to this.
Geiger-Muller Counter
You can use this apparatus to detect and quantify the emission rate of α- or β-particles. A cathode is a cylindrical metal tube with a wire running through its center. The tube is pressurized with argon gas (0.1 atm). Between the electrodes, a potential difference of around a thousand volts is applied. Argon atoms become ionized when α- or β-particle enters the tube through the mica glass.
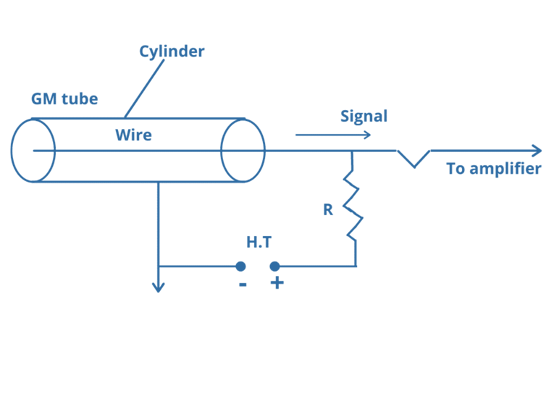
Argon ions (Ar+) are attracted to the cathode, while electrons are attracted to the anode. As a result, the circuit is completed for a fraction of a second by a brief pulse of electrical current between the electrodes. A particle is counted as it enters the tube with each individual electrical pulse. The intensity of radioactivity is proportional to the number of such pulses measured by a radioactive material each minute.
Scintillation Counter
Rutherford detected and counted α-particles using a spinthariscope. α-particles. were released from the radioactive material at the wire’s end. The zinc sulphide screen glowed brightly as each particle smashed into it. The eye-piece revealed these brief illuminations, or scintillations. This apparatus allowed for the counting of 50–200 α-particles per second.
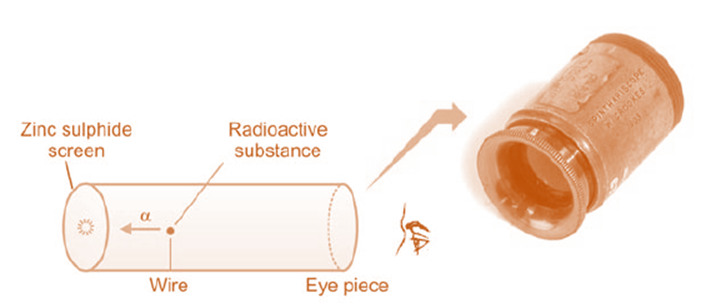
A modern scintillation counter, which is commonly used for measuring α- or β-particles, is based on the same principle. A crystal of sodium iodide with a trace of thallium iodide is used in place of the zinc sulphide screen. A little vial containing the radioactive material is lowered into a well carved into the crystal. Scintillations were created when the sample’s radiation collided with the crystal wall. When these hit a photoelectric cell, they trigger a brief electrical discharge. A mechanical counter keeps track of this information. A scintillation counter of this type can detect and record up to a million counts of radiation every second.
Film Badges
The components of a film badge are photographic film and a plastic holder. Radiation can make the silver in photographic film darken. A high-powered microscope is used to examine the developed film. Black particles can be seen where α- or β-particles have passed through the film. These are quantifiable particles. Both the kind of radiations and strength can be determined in this way. The photographic film is uniformly darkened by γ-radiation, though. The level of darkening is indicative of the dose of radiation.
Workers in radioactive environments should wear film badges to track their radiation exposure levels. At regular intervals, the film in the badge is developed to reveal how much radiation the wearer has taken in.
Units of Radioactivity
The standard unit of radioactivity (i.e. rate of disintegration) is Curie (c). A curie is a quantity of radioactive material decaying at the same rate as 1 g of Radium (3.7 × 1010 dps). Rutherford is a more recent unit.
1 Rutherford = 106 dps
The S.I. unit is Becquerel 1 Bq = 1 dps
Radioactive Decay
Radioactivity is a nuclear property, as proposed by Rutherford and Soddy (1903). A radioactive atom has an unstable nucleus. It disintegrates or decays through the spontaneous emission of α- or β-particle. The proton-neutron ratio of neutron shifts as a result, creating a more stable atomic structure. The former/original is referred to as the parent nucleus, while the latter/product is known as the daughter nucleus.
There are following types of radioactive decay:
Alpha (α)-decay
The process of a radioactive nucleus decaying via the emission of α-particle (α-emission) is known as α-decay. The alpha particle has a mass of four and a positive charge of two. Assuming that the parent nucleus has atomic number Z and mass M, the daughter nucleus will have
Atomic mass = M – 4
Atomic number = Z – 2
Thus an α-emission reduces the atomic mass by 4 and atomic number by 2
The nucleus of helium, or 42He, is an alpha particle. When a nucleus undergoes alpha decay, it changes into a different nucleus and releases an alpha particle in the process. When 23892U decays into 23490Th, for instance, it is an example of alpha-decay.
Now, 42He contains two protons and two neutrons. Therefore, after emission, the emitting nucleus’ mass number decreases by four and its atomic number decreases by two. So, to express the change from an AZX nucleus to an A-4Z-2X nucleus, we have
AZX → A-4Z-2X + 42He
where
AZX is the parent nucleus and
A-4Z-2X is the daughter nucleus.
The lightest elements do not undergo alpha decay; only the heaviest ones do. The element’s nucleus must be either sufficiently large or sufficiently unstable to undergo fission-like changes on its own. In such elements, this decay process predominates over all others. The nucleus’s alpha particles typically travel at a speed of about 5% the speed of light and have an energy level of about 5 MeV. Because electrons are absent from alpha particles, they have a charge of +2. This charge, combined with the alpha particle’s relatively large mass, causes it to interact with its environment in a way that quickly drains all of its energy. They can be stopped in their tracks by just a few centimeters of air.
Beta (β)-decay
A radioactive nucleus undergoes beta-decay when it disintegrates via beta-particle emission (β-emission). The nucleus does not have any free electrons or beta particles. At the time of emission, a neutron is converted into a proton, creating the element. This causes the nucleus to gain one positive charge. If the loss of β-particle from an atom’s nucleus, the atom’s mass will remain the same. The daughter nucleus of a parent nucleus with mass number Z and atomic mass M will have
Atomic mass = M
Atomic number = Z + 1
Lead-214 decays to bismuth-214, is an example of β-decay.
It’s important to note that an isobar is created when β-emission occurs. For this reason, 21482Pb and 21483Bi are isobaric, despite having different atomic numbers but the same mass number (82 and 83).
The production of an isotope requires one α-emission and two β-emissions Let’s think about the changes that are about to occur.
Gamma decay
Energy levels in atoms are well-known. The energy levels of a nucleus are comparable. In order to return to a less energetic ground state, an excited nucleus can release electromagnetic radiation. In addition, the nucleus has energy states with a difference in MeV. Therefore, the photons emitted by the nuclei are known as Gamma rays and have energies of MeV. Most radioactive particles, after emitting alpha or beta particles, leave the daughter nucleus in an excited state. The emission of a single or multiple gamma rays brings this daughter nucleus to its ground state.
The beta decay of 6027Co produces 6028Ni. The daughter nucleus (6028Ni) is in its excited state. By emitting two gamma rays with energies of 1.17 MeV and 1.33 MeV, this excited nucleus returns to its ground state.
Watch this interesting video with short description on alpha, beta, and gamma rays.
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.
- https://www.toppr.com/guides/physics/nuclei/radioactivity-types-of-radioactive-decay/
- https://byjus.com/physics/radioactivity-alpha-decay/

