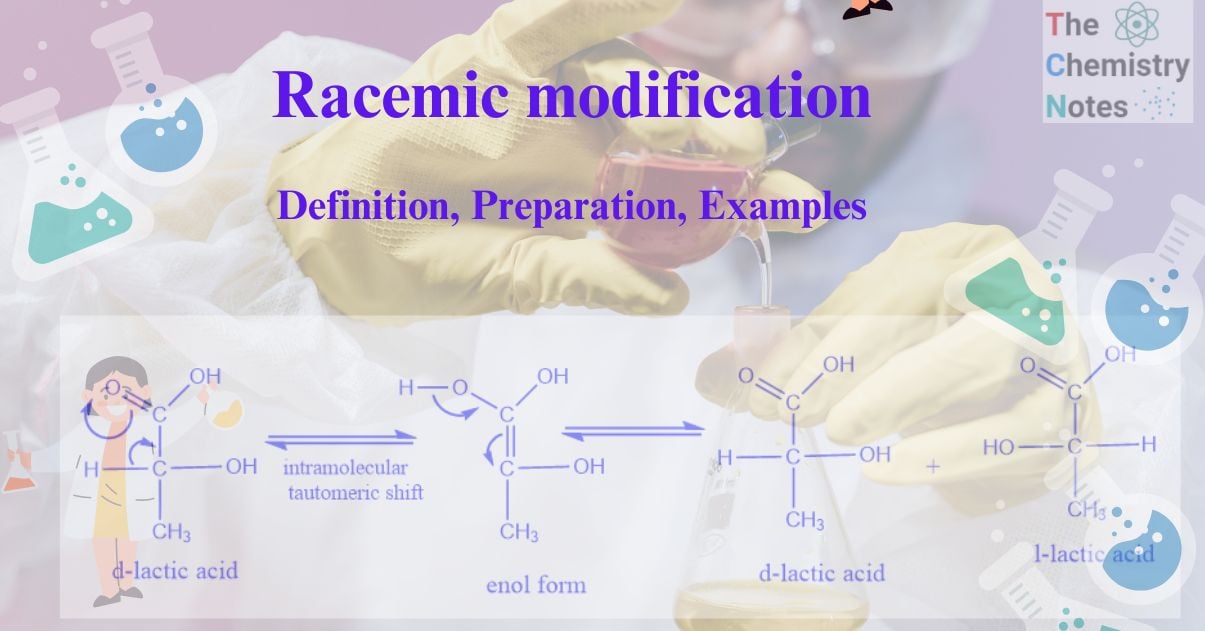
Racemic modification is the mixture of two enantiomers, substances with mirror-image or dissymmetric chemical structures in equal amounts. Racemic modification refers to the equimolar mixture of two enantiomers. It is also known as recemic mixture or racemate.
Each enantiomer rotates the plane of polarization of plane-polarized light by a specific angle. When two enantiomers are mixed in equal parts, their dextro and leavo rotatory optical activity is canceled by the equal and opposite rotation of the individual enantiomers. As a result, the racemic mixture is optically inactive. External compensation is considered to be the cause of this type of optical inactivity. The recemic modification is denoted by dl or (±).
For example
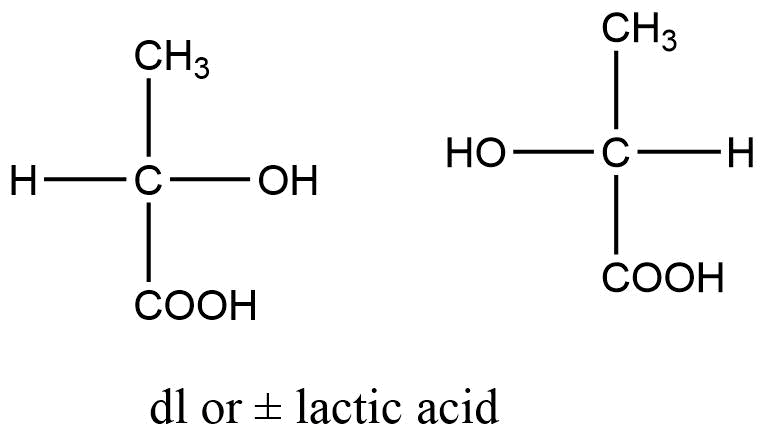
Preparation of racemic modification
I. By mixing of enantiomers
A racemic modification can be produced by mixing two equal amounts of two enantiomers. This method is commonly used when comparing racemic modifications generated through another process to the available enantiomers of the same species. The products will have the same melting points in both situations.
II. By assymetric synthesis
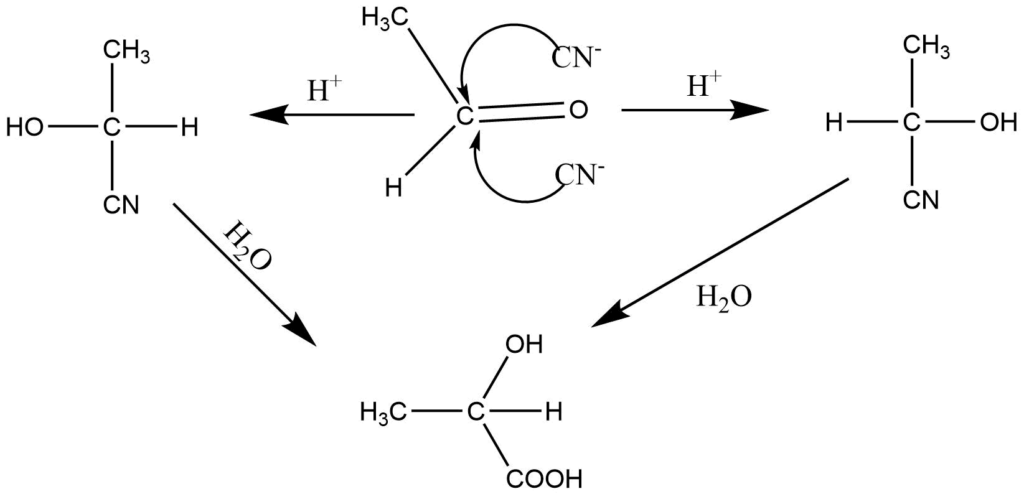
Racemic modification occurs when an asymmetric molecule is generated from a symmetrical compound in the absence of an optically active substance. For instance, acetaldehyde and HCN are used to produce lactic acid. Attacking nucleophiles from above and below the plane of the acetaldehyde molecule is equally possible. Then the protonation of carbonyl oxygen occurs to produce lactronitrile. The lactronitrile on hydrolysis yields dl lactic acid. This is referred to as asymmetric synthesis.
III. By racemization
The process of converting an optically active compound (dextro rotatory or laevo rotatory) into a racemic modification is known as racemization. As a result, in racemization, we start with an optically active substance and end up with an optically inactive substance. In this case, either a dextro or a Laevo rotatory molecule is directly transformed into an inactive racemate state. For example, if a compound is dextro rotatory, racemization would include converting half of its dextro form into laevo form, resulting in an optically inactive combination due to the presence of equal amounts of two enantiomers.
Racemization can occur in several ways, but it is expected to produce a symmetrical intermediate during the reaction. Some examples with various mechanisms are given below.
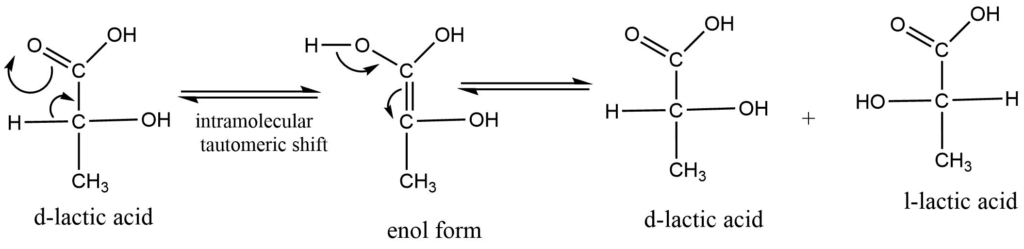
a. Intermolecular rearrangement
Racemization occurs as a result of an intramolecular rearrangement brought on by heat, light, or chemical agents such as acids or alkalis. The optically active chemicals are temporarily in equilibrium with this rearranged product as a result of this rearrangement. The latter lacks an asymmetric center, therefore when it returns to the asymmetric center, both the d and l forms are produced in the same amount. for example: racemization of lactic acid:
b. Thermal recemization (mechanism involving free radical)
In this process, the action of heat causes one of the bonds binding an asymmetric carbon atom to break down, releasing a radical, and reforming the bond results in racemic modification. A distillation of phenyl chloride, for example, yields dl phenyl chloride. The carbon-chlorine bond is thought to be broken homolytically. It demands a high temperature. Such recemizations are quite uncommon.
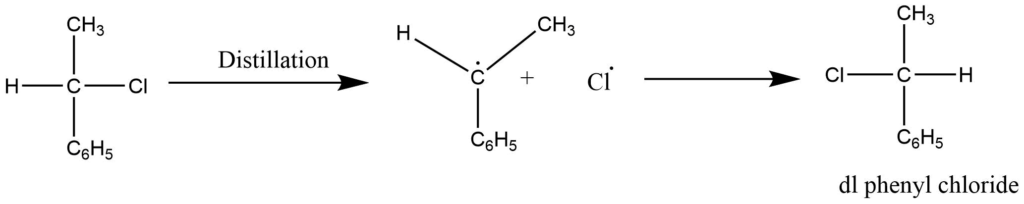
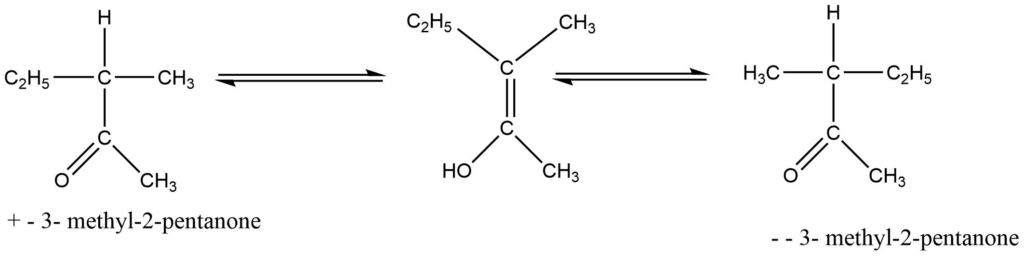
c. By anion formation ( Mechanism involving carbanion)
If a carbonyl group connected to a chiral center contains one hydrogen atom in that asymmetric carbon atom, the hydrogen atom is ionizable. Alkali generally causes these molecules to racemize. In other terms, it is a base-catalyzed process. In this process, alkali extracts the hydrogen-producing carboanion, which quickly transforms into the symmetrical optically inactive intermediate, the enol form of the molecule. The interconvertible keto-enol compounds result in racemic modification. For example: the recemization of 3-methyl-2-pentanone
d. By cation formation (Mechanism involving carbonium ion)
In this method, the carbonium ion is generated by the temporary removal of a group with a pair of electrons in the presence of Lewis to give racemic modification. For example: Recemization of phenyl chloride in presence of antimony pentachloride.
C6H5CHClCH3 + SbCl5 ⇌ C6H5C+HCH3 +SbCl6– ⇌ C6H5CHClCH3 + SbCl5
(+)- (-)-
IV. Walden inversion
If the leaving group is joined to an asymmetric carbon atom during the substitution nucleophilic bimolecular process, the product molecule will take on the opposite configuration to the starting compound. In other words, the configuration will be inverted.
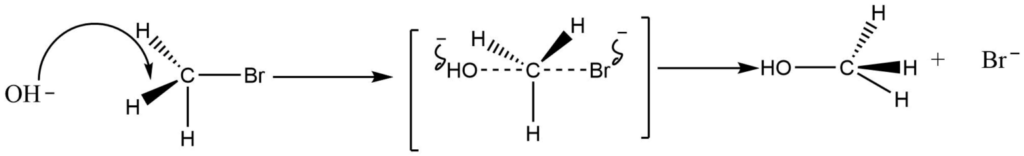
V. Recemization through rotation around bonds
When it comes to configurational isomers, racemization also occurs as a result of rotation around the single bond. When biphenyls are substituted with steric groups at the proper location, optical activity is produced. Most of these enantiomers are racemized by thermal processing, which aids the nonplanar enantiomers in crossing the planar transition state. A pivoted bond connects the two phenyl groups in biphenyl. It is possible to have cisoid and transoid transformations if groups like “a” and “b” are substituted in the ortho position.
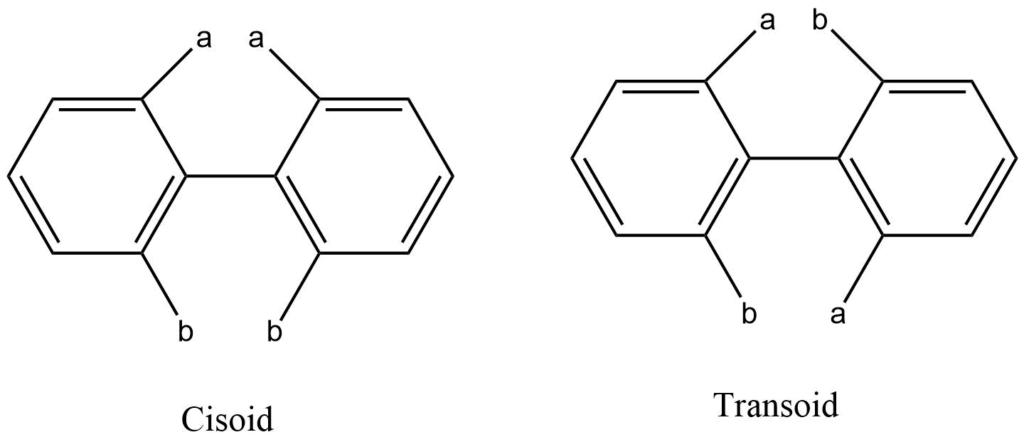
Racemization occurs more readily through transoid conformation because the cisoid has higher energy than the transoid. The bulker groups in the ortho position cause a higher energy barrier so that the enantiomers can be easily separated at room temperature. Atropisomers are a type of isomers that can be isolated as the result of constrained rotation around a single bond, and the phenomenon is referred to as atropisomerism.
Racemization of amino acid
An acid or a base can catalyze the racemization of an amino acid. However, base particularly catalyzes it.
Racemization of free amino acids is challenging. It has been discovered that free amino acids do not racemize as quickly as amino acid derivatives. Several factors increase the rate of racemization in amino acids. An increase in the electronegativity of R and a decrease in the negative charge on the carboxylate group of amino acids enhance the rate of racemization. Similar to this, the rate of racemization is increased by substituting hydrogen on the amino group with electronegative atoms or groups like acyl groups. For example, Reaction of benzoyl chloride with glycine readily produce benzoyl glycine.
C6H5COCl + H2NCH2COOH → C6H5COHNCH2COOH + HCl
Racemization of amino acids is very important in the field of research due for following reasons:
- It helps the study of the origins of life.
- It assists in determining the age of fossils.
- The loss of starting material can be prevented by racemization.
- Amino acids are the building blocks of proteins and peptides. As a result, effects that minimize undesired racemization of amino acids can be performed during protein synthesis.
Suggested video
References
- K.R palak,2017, Stereochemistry. Pairavi Prakashan.
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- https://solutionpharmacy.in/racemic-modification/.
- https://www.britannica.com/science/racemate
- https://sites.ualberta.ca/~csps/JPPS1(1)/A.Mitchell/racemicview.htm
