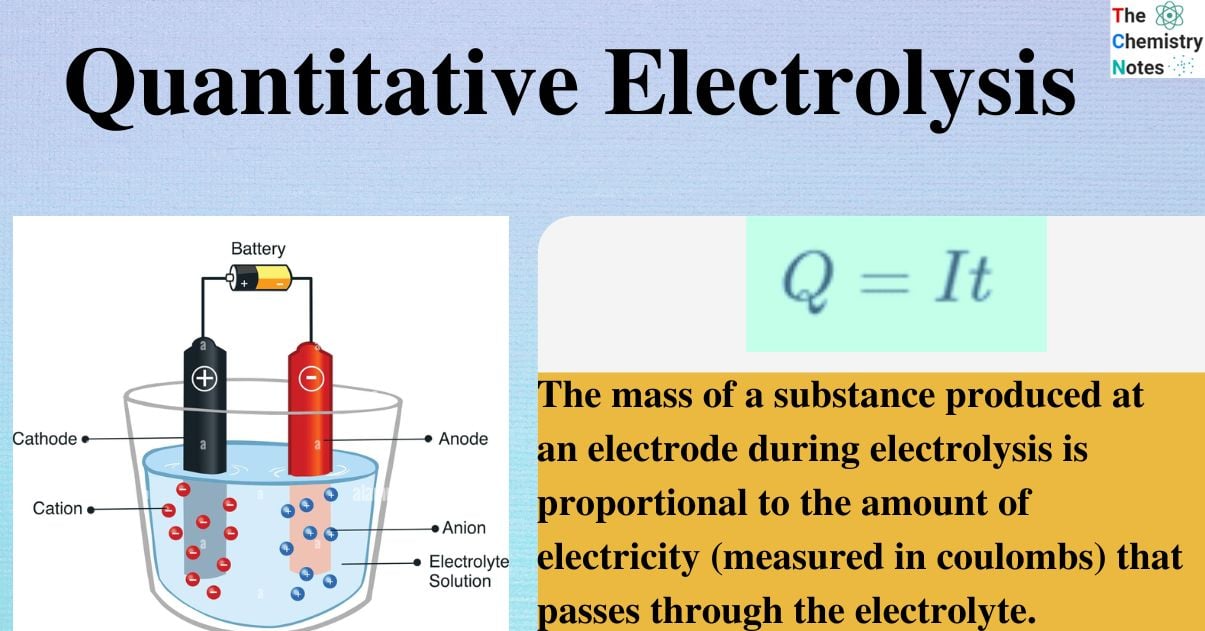
Quantitative Aspects of Electrolysis was first acknowledged by Michael Faraday. Electrolysis is a process in which an electric current drives a chemical reaction across electrodes. The medium in which electrolysis occurs is known as the electrolyte, which can be an ionic solution or molten mass.
Quantitative Aspects of Electrolysis
The amount of material deposited at the electrode during electrolysis is proportional to:
- the time it takes for a constant electric current to pass through the electrode;
- and the strength of the electric current.
We get the relationship by combining current and time.
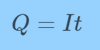
Where;
Q = charge (in coulombs, C)
I = current (in amperes, A)
t = time (in seconds, s)
The mass of a substance produced at (or removed from) an electrode during electrolysis is proportional to the amount of electricity (measured in coulombs) that passes through the electrolyte.
The amount of electricity is frequently expressed in terms of the Faraday unit (symbol F). 1 Faraday represents the amount of electric charge carried by 1 mole of electrons or 1 mole of singly charged ions. It has a value of 96,500 Cmol-1 (to 3 significant figures).
Faraday’s Laws of Electrolysis
M. Faraday discovered certain relationships between the amount of electricity passing through an electrolyte and the amount of any material liberated at the electrode while researching the phenomenon of electrolysis. The amount of electricity is the product of the current strength and the time it is passed. Hence, Faraday’s findings can be summarized in the form of the two laws of electrolysis, as follows.
First Law of Electrolysis
The First Law of Electrolysis states that “the amount of chemical reaction or change so produced at any electrode during electrolysis by a current is proportional to the quantity of electric charge passing through an electrolytic cell”.
Mathematically, it can be expressed as:
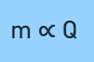
where ‘m’ is the mass of a substance (in grams) deposited at the electrode and ‘Q’ is the amount of charge (in coulombs) passed through it
On removing the proportionality constant, the equation becomes:
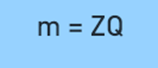
where ‘Z’ is the electrochemical equivalent or proportionality constant
It can also be defined as the mass of a substance deposited on an electrode on passing 1 coulomb of charge during electrolysis. The unit of Z is grams per coulomb (g/C).
Second Law of Electrolysis
The second Law of Electrolysis can be stated as “the amount of different substances released by the same quantity of electricity passing through the solution is proportional to their chemical equivalent weights”.
Therefore it can be expressed as;

where ‘w’ is the mass of the substance and ‘E’ is the equivalent weight of the substance. Or, it can also be expressed as,
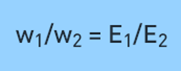
Example of Calculation
During the electrolysis of silver nitrate solution, silver is deposited at the cathode:
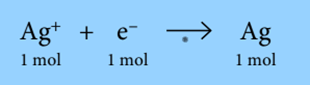
1 Faraday of electricity (96 500C) is required to deposit 1 mole of silver. During the electrolysis of copper (II) sulfate solution, copper is deposited at the cathode:
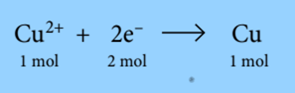
The equation shows that 2 moles of electrons are needed to produce 1 mole of copper from Cu2+ ions. So, it requires 2 Faradays of electricity (2 × 96500C) to deposit 1 mole of copper. During the electrolysis of molten sodium chloride, chlorine is produced at the anode:
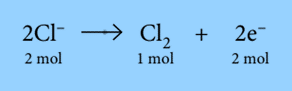
The equation demonstrates that when 1 mole of chlorine gas is created from 2 moles of Cl– ions, 2 moles of electrons are released. Therefore, to produce 1 mole of Cl2, 2 Faradays of electricity (2 * 96500 C) are needed.
In the course of electrolysis of an aqueous solution of sulfuric acid or aqueous sodium sulfate, oxygen is produced at the anode.
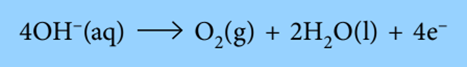
According to the equation, the formation of 1 mole of oxygen gas from 4 moles of OH– ions releases 4 moles of electrons. Therefore, 1 mole of O2 is created using 4 Faradays of electricity (4 * 96500 C).
Calculating the mass of material
The mass of material deposited at an electrode and the volume of gas produced at an electrode can both be calculated using the value of F.
Calculate the mass of lead deposited at the cathode during electrolysis when a current of 1.50A flows through molten lead (II) bromide for 20.0min.
[(Ar value: [Pb] = 207; F = 96500Cmol–1)]
Step 1: Write the half-equation for the reaction.
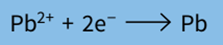
Step 2: Find the number of coulombs required to deposit 1 mole of product at the electrode.
2 moles of electrons are required per mole of Pb formed = 2F = 2 × 96500 = 193000 C mol–1
Step 3: Calculate the charge transferred during the electrolysis.
Q = I × t = 1.50 × 20 × 60 = 1800C
Step 4: Calculate the mass by simple proportion using the relative atomic mass.
193000C deposits 1 mole Pb, which is 207g Pb
So, 1800C deposits (1800 / 193000) * 207 = 1.93g Pb
Calculating the volume
Calculate the volume of oxygen produced at r.t.p. when a concentrated aqueous solution of sulfuric acid, H2SO4, is electrolyzed for 30.0 min using a current of 0.50 A.
(F = 96500 C mol–1; 1 mole of gas occupies 24.0 dm3 at r.t.p.)
Step 1: Write the half-equation for the reaction.
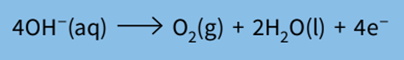
Step 2: Find the number of coulombs required to produce 1 mole of gas.
4 moles of electrons are released per mole of O2 formed = 4F = 4 × 96500 = 386000Cmol–1
Step 3: Calculate the charge transferred during the electrolysis.
Q = I × t = 0.50 × 30 × 60 = 900C
Step 4: Calculate the volume by simple proportion using the relationship 1 mole of gas occupies 24.0 dm3 at r.t.p.
386000C produces 1 mole O2, which is 24dm3 O2
So, 900C produces (900 / 386000) × 24.0
= 0.0560dm3 O2 at r.t.p.
Calculating the Avogadro constant by an electrolytic method
The Avogadro constant, L, is the number of specified particles in 1 mole.
By calculating the charge associated with 1 mole of electrons, we can use an electrolytic method to determine the value of the Avogadro constant.

We can calculate the charge of the electron through experimentation.
As a result, this shows us that the charge on the electron is approximately 1.60 × 10–19 C.
Finding the charge on 1 mole of electrons
A straightforward electrolytic experiment can thus be used to determine the charge on 1 mole of electrons.
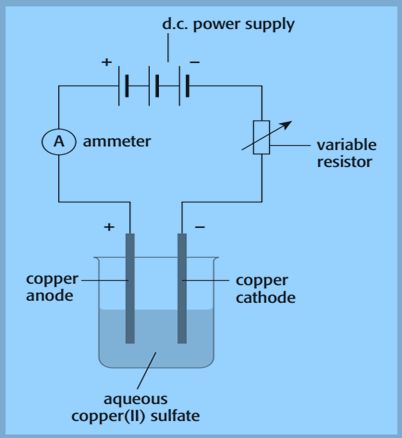
The steps are as follows:
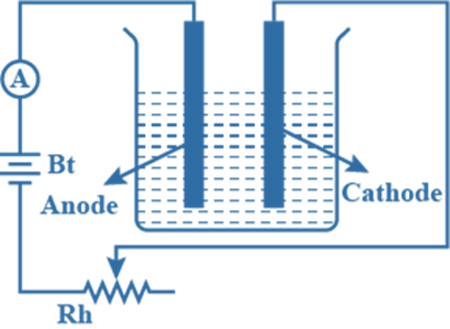
- weigh the pure copper anode and pure copper cathode separately
- arrange the apparatus as shown in the figure. Similarly, the variable resistor is used to keep the current constant b
- pass a constant electric current for a measured time interval
- remove the cathode and anode and wash and dry them with distilled water and then with propanone
- Finally, reweigh the cathode and anode.
Due to the deposit of copper, the cathode gains mass. Because copper enters the solution as copper ions, the mass of the anode decreases. It measures the anode’s loss in mass. This is preferred because the copper does not always stick to the cathode very well.
Equivalent Weight
Faraday’s Second Law of Electrolysis states that a substance’s equivalent weight is equal to the product of its atomic weight and valency.
Therefore, it can be written as
Atomic Weight/Valency = Equivalent Weight
It will be obvious that the metals Ag, Cu, and Al collect at the cathode if we take a solution of the electrolytes AgNO3, CuSO4, and Al (NO3)3 and simultaneously apply a potential of voltage through them. With their equivalent masses, their masses are proportionate.
Corresponding Mass = Element Atomic Mass / Element Valency
According to Faraday, if 1 Faraday (or 96,500 Coulombs) is passed through them, hence we obtain
The equivalent masses of Ag (108/1 = 108 g),
Equivalent masses of Cu (63.5-2 = 31.75 g), and
Equivalent masses of Al (27/3 = 9 g).
References
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_ChemPRIME_(Moore_et_al.)/17%3A_Electrochemical_Cells/17.07%3A_Quantitative_Aspects_of_Electrolysis
- https://www.savemyexams.co.uk/dp/chemistry_hl/ib/16/revision-notes/19-hl-redox-processes/19-1-electrochemical-cells/19-1-4-quantitative-electrolysis/
- https://study.com/skill/learn/calculating-quantitative-electrolysis-explanation.html#:~:text=Electrolytic%20cells%20can%20be%20used,l%20c%20o%20u%20l%20).
- https://collegedunia.com/exams/quantitative-aspects-of-electrolysis-definition-formula-factors-and-sample-questions-chemistry-articleid-1766#wei
- https://www.toppr.com/ask/en-np/content/concept/quantitative-electrolysis-260646/
