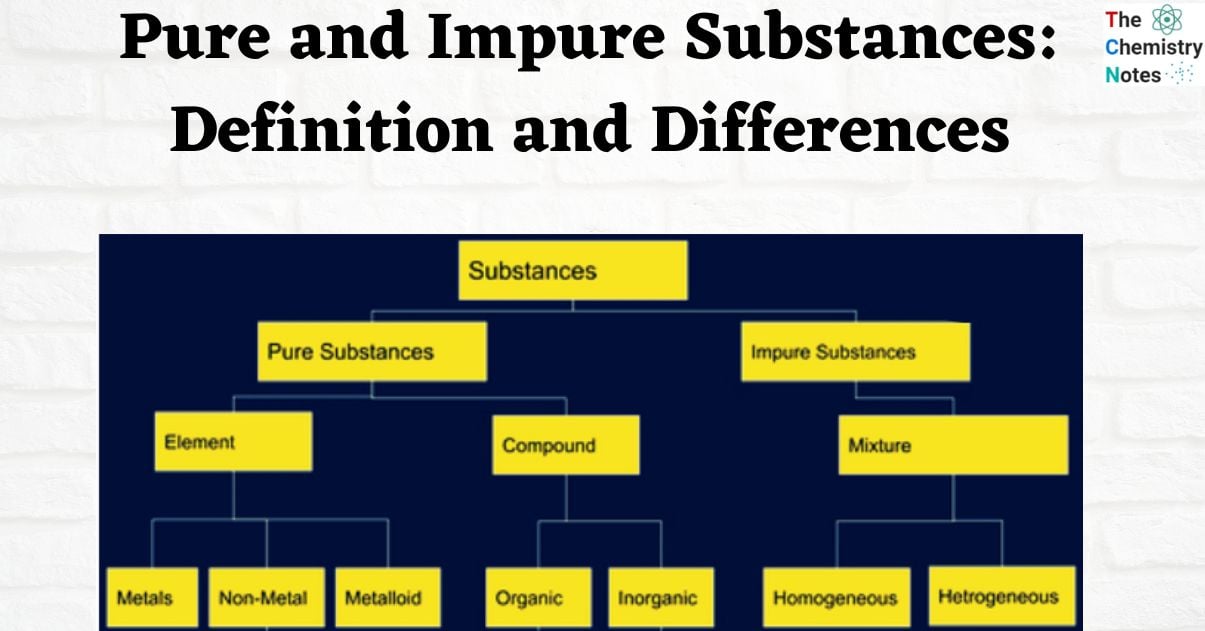
A substance is a type of matter that has specific features and compositions. Substances are made up of atoms and molecules. Each substance has a specific weight and volume. It cannot be separated into distinct types of matter through physical processes. All elements and pure compounds are substances. Substances are usually classified into two types: pure and impure substances.
Pure Substances
A pure substance has the same chemical composition every time. It contains no impurities or pollutants. A pure material is sometimes known as a “chemical substance.” Pure substance can be further categorized into two types:
Elements
An element is a pure material made up of a single type of atom. It cannot be broken down or changed into new compounds through any physical or chemical process. Metals, nonmetals, and metalloids are the three main types of elements. Copper and platinum, for example, are elements.
Graphite is a pure substance that can be found in the lead of a pencil. When you look closely at the lead of a pencil, you will notice that it is composed entirely of carbon atoms. Graphite is a mineral form of carbon that occurs naturally and is a pure substance.
Compounds
A compound is a form of a pure substance that is made up of only one type of molecule. Compounds are produced when two or more elements combine chemically in a specific ratio. This type of substances can be broken down using chemical techniques.
When we examine a molecule at the molecular level, we see that atoms are bound together in a precise configuration.
Water, for example, is formed when hydrogen and oxygen mix. Both hydrogen and oxygen are elements.
State of The Pure Substances
A phase is a specific molecular arrangement that is uniform throughout. At the same time, it may be distinguished from others by plainly discernible border surfaces.
A substance may be found in multiple phases, each with a unique molecular structure. Carbon, for example, may appear as graphite or diamond in the solid phase. Pure substance occur in three states which are discussed below:
Solid: All molecules within the solid-state arrange themselves in a three-dimensional pattern called a lattice. These molecules are immobile, but they fluctuate around their equilibrium position. Examples of substances in the solid state are gold, silver, and platinum.
Gas: The molecules that exist in the gaseous state are separated from one another. Furthermore, there is no molecular order. Gas molecules can move freely and continually collide with one another. Examples of substances in the gaseous state are oxygen, hydrogen, and argon.
Liquid: The molecular dispersion in the liquid state is nearly identical to that in the solid state. The only alteration that has occurred is the molecular structures. They do not stay in a fixed position. Examples of substances in the liquid state are mercury, hydrochloric acid, and sulfuric acid.
Properties of Pure Substance
The pure substance’s properties represent the distinct chemical and physical traits to characterize and differentiate a material from others. Some properties of pure substances are discussed here:
Physical Properties: Pure substances have physical properties like boiling point, melting point, density, color, etc. These characteristics are consistent throughout the bulk, sample size does not matter.
Chemical Properties: These properties describe how a chemical behaves when it interacts with other substances. Reactivity, flammability, and corrosiveness are a few examples.
Intensive Properties: These traits are constant regardless of the amount of material present. Density, boiling point, and melting point are all examples.
Extensive Properties: These qualities are affected by the amount of drug present and change with it. Mass, volume, and heat capacity are a few examples.
Impure Substances
Pure substances are composed of atoms or molecules of various sorts. They are not always the same composition. They are also referred to as mixes. Using different separation procedures, impure chemicals can be turned into pure substances. Impure substances can be further categorized into two different types that are discussed here:
Heterogeneous Mixtures: A heterogeneous mixture is the combination of two or more pure substances that have not been chemically mixed and maintain their inherent chemical characteristics. A mixture with a non-uniform composition is referred to as a heterogeneous mixture. Mixture of oil and water is the example of heterogeneous mixture.
Homogeneous Mixtures: It is a mixture where the constituents are uniform, its composition is same and appears a single substance. For example common salt and water makes salt solution which does not change its appearance prior to the mixing.
Properties Of Impure Substances
The attributes of the final combination are an average of the constituents’ properties.
Physical change causes formation.
It might be homogeneous or heterogeneous. Substances differ in their composition.
Difference Between Pure And Impure Substances
| Difference | Pure Substance | Impure Substance |
|---|---|---|
| Meaning | Pure substances are composed of a single element or compound. | Several elements and compounds together form an impure substance. |
| Categories | They are categorized into solids, liquids, and gases. | They are categorized as heterogeneous and homogeneous. |
| Properties | Physical properties and chemical properties are constant | Physical properties and chemical properties can vary |
| Purity | They are 100 percent pure | Their purity is compromised |
| Seperation | Cannot be separated by using the physical methods | Can be separated using the physical techniques |
| Examples | Gold, Diamond, and Pure water | Oil and water, salt and sugar |
Video on Pure and Impure Substance
References
- https://unacademy.com/content/nda/study-material/chemistry/differences-between-pure-and-impure-substances/
- https://byjus.com/question-answer/difference-between-a-pure-and-impure-substances-b-homogeneous-and-heterogeneous-substances/
- https://www.geeksforgeeks.org/pure-and-impure-substances/
- https://byjus.com/chemistry/pure-substances-and-mixtures/

