Regarding the prediction of the type of polymerization, before anything else, it’s critical to recognize the monomers’ primary functional groups. You may encounter a variety of polymer-related problems to solve:
- predict the kind of polymerization reaction that will occur for a specific monomer or pair of monomers.
- determine the polymer’s repeat unit from a given monomer or pair of monomers.
- determine the type of polymerization reaction that produces a given section of a polymer molecule
- determine which monomer(s) are present in a specific polymer molecule section.
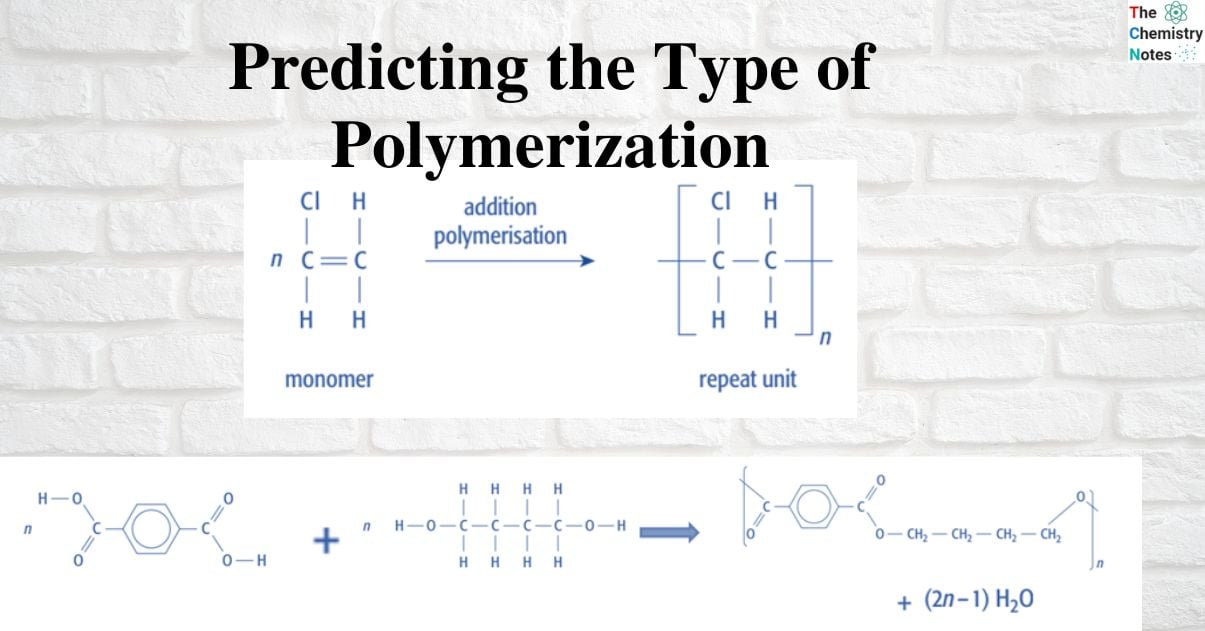
Predicting the type of polymerization reaction for given monomer(s)
Addition polymers
- A monomer with a C=C double bond will go through addition polymerization.
- One type of monomer is used to create numerous addition polymers, such as poly(propene) from propene monomers.
- However, co-polymers can also be made by combining different unsaturated monomers, such as H2C = CH2 and H2C = CHCOOH.
Condensation polymers
- Search for two functional groups that will interact with one another during condensation polymerization and release a small molecule as a byproduct.
- These two functional groups can also be found within the same molecule, as in the case of nylon 6,6, or at either end of two different monomers, as in the case of poly (lactic acid).
The following functional groups are typically involved in condensation polymerization:
- amines (-NH2) and carboxylic acids (-COOH) produce a polyamide and H2O
- amines (-NH2) and acyl chlorides (-COCl) produce a polyamide and HCl
- carboxylic acids (-COOH) and alcohols (-OH) produce polyester and H2O
- acyl chlorides (-COCl) and alcohols (-OH) produce a polyester and HCl.
Deducing the repeat unit of a polymer for given monomer(s)
Addition polymers
Simply turn the C = C double bond of a monomer into a C – C single bond, and then display the bonds on either side of the two C atoms that would make up the next link in the chain:
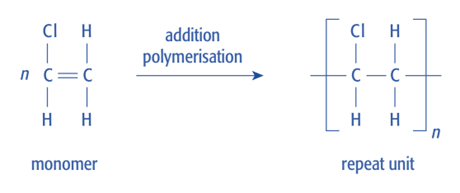
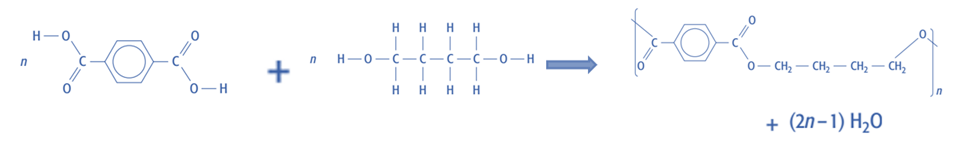
Condensation polymers
- Draw the product formed when two monomers with two reactive functional groups react together.
- Then remove the atoms at the ends that would be lost if those groups were to react with another two monomers.
Deducing the type of polymerization reaction for a given section of a polymer chain
Addition polymers
In the actual chain of carbon atoms that makes up the polymer’s “backbone,” addition polymerization produces repeat units without any functional groups.
Example: Poly(phenylethene)
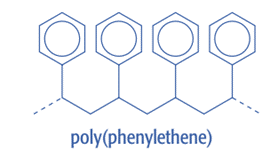
Be aware that functional groups, like the nitrile group, since CN, may be present on the sidechains. Though, the “backbone” of polymers is typically made up of a chain of carbon atoms.
Condensation polymers
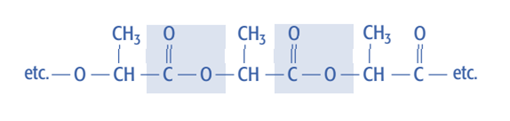
Condensation polymerization produces polymers with amide (-CONH-) or ester (-COO-) links in the polymer chain’s “backbone.” For example:
Identifying the monomer(s) present in a given section of a polymer chain
After determining whether the polymer was created through condensation or addition polymerization, you can divide the polymer chain into its repeat units.
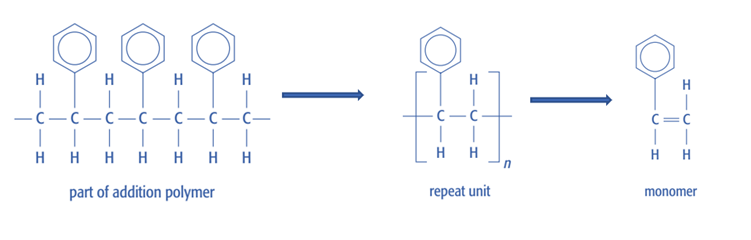
Addition polymers
Put the C-C double bond back into the monomer when using an addition polymer:
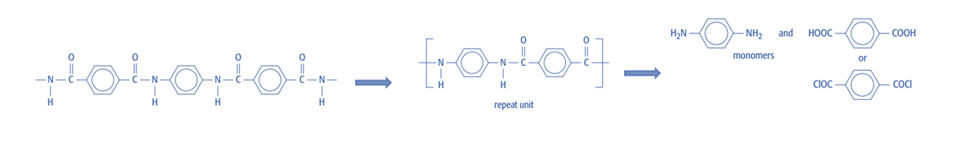
Condensation polymers
Identifying the atoms from the small molecules released during the polymerization reaction is necessary when creating condensation polymers so that they can be substituted on the reactive functional groups in the monomers.
References
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision- notes/7-organic-chemistry-a-level-only/7-7-polymerisation-a-level-only/7-7-4- predicting–deducing-the-type-of- polymerisation/
- Hill and Holman (2011) Chemistry in Context, Sixth edition, Nelson Thornes
- Clugston and Flemming (2000) Advanced Chemistry, Oxford University Press
