
Precipitation titration is the type of titration that involves precipitation reactions.It is based on the production of precipitation at the end point of the titrimetric analysis. In a precipitation titration, the titrant and analyte combine to create a precipitate, an insoluble material. It continues until all of the analytes have been consumed. Examples of such titrations are often limited to those involving the precipitation of silver ions with anions like halogens or thiocyanate (SCN–). The lack of appropriate indicators is one reason for the limited application of such reactions. Sometimes the response rate is too slow, especially when titrating dilute solutions. Once the equivalency point is approached and the titrant is gradually added, a high degree of supersaturation does not occur and the precipitation rate may be quite slow. Additionally, due to the co-precipitation reaction, it is usually difficult to determine the composition of the precipitate.
Halide ions and thiocyanate ions can be quantitatively precipitated by silver nitrate from their solutions, and these precipitation reactions provide the basis for the titrimetric determination of the ions.
AgNO3 + X– → AgX↓ + NO3–
Where, X= Cl–, Br–, I– or SCN–
For example, The concentration of the chloride ion present in the unknown solution can be determined by titrating with a solution of a known concentration of silver nitrate. In this titration, silver chloride precipitates out as a white substance. The amount of silver ions utilized to calculate the endpoint is equivalent to the number of chloride ions that were initially present.
Reaction involved in thid titration is as follows;
AgNO3 + Cl– → AgCl↓ + NO3–
Different methods of argetometric titration
Mohr’s method (Color precipitate formation)
Karl Friedrich Mohr, a German chemist, proposed this approach. Because of this, this technique is known as Mohr’s approach. It is a method of direct titration. This method uses chloride ion solution as the analyte and silver nitrate as the titrant. Potassium chromate is used as an indicator. An indicator is a diluted solution of a suitable chemical that precipitates in an intense color when the reagent is introduced from a burret. For example, in Mohr’s method of argentometric titration of Cl– involving the precipitation process, the endpoint is determined by adding a little amount of potassium chromate as an indicator.
For example. Cl– ions precipitate as AgCl when NaCl with potassium chromate as an indicator is titrated with AgNO3 solution. Excess Ag+ ions interact with CrO4— present as an indicator after all of the Cl– precipitates as AgCl, producing a brick-red precipitate of silver chromate.
AgNO3 + NaCl → AgCl ↓ (white ppt.)+ NaNO3
2AgNO3 + K2CrO4 → Ag2CrO4↓( brick red ppt.) + 2KNO3
This is an example of fractional precipitation of two sparingly soluble salts, AgCl and Ag2CrO4. Ag2CrO4 is more soluble than AgCl. When silver ions are introduced to a solution with a high concentration of Cl- ions and a low concentration of CrO4— ions, AgCl will precipitate out first. Ag2CrO4 won’t form until the concentration of Ag+ ions rises to a level that exceeds the Ksp (solubility product) of silver chromate. As a result, the precipitation of the indicator occurs at or near the titration equivalence point.
Volhard’s method (Colour solution formation)
In 1874, German chemist Jacob Volhard introduced this technique for the first time. This method uses silver ions to measure halide (F, Cl, Br, I) ions as well as anions like phosphate and chromate in an acidic media.In Volhard’s technique for argentometric titration of Cl–, the excess of unreacted Ag+ ions is determined by titrating the mixture against a standard thiocyanate solution while utilizing the Fe(III) ion as an indicator.
Ag+ + SCN– → AgSCN
Fe3+ + SCN– → FeSCN2+ (red)
Fajan’s method (Adsorption indicator)
This method was first introduced by American chemist Kazimierz Fajan. So, it is known as fajan’s method. An adsorption indicator is a chemical that is adsorbed on a precipitate’s surface and changes the color of the precipitate. This was developed by K. Fajans. It is a powerful indicator for determining the endpoint in a precipitation titration. In this method, the precipitate adsorbs these indicators, and during the adsorption process, a change occurs in the indicator that leads to a substance of different color. As a result, they are known as adsorption indicators.
There are two different types of adsorption indicators. They are:
i. Acid dyes : Fluorescein series ( e.g., Fluorescein and eosin which are utilized as sodium salt).
ii. Basic dyes: e.g., Rhodamine series. (e.g., rhodamine-6-G which is applied as halogen salts).
Mechanism of action of adsorption indicators
The mode of action of the adsorption indicators is based on the properties of colloidal particles. The precipitate AgCl adsorbs the Cl– ion when a chloride solution is titrated with a solution of AgNO3 (a precipitate tends to adsorb its ions). This is known as the primary adsorption layer, and it is retained by the secondary adsorption of oppositely charged ions in the solution. When the equivalence point is achieved, there is an excess of Ag+ ions, which displaces Cl– ions from the primary layer and holds nitrate ions in the secondary adsorption layer.
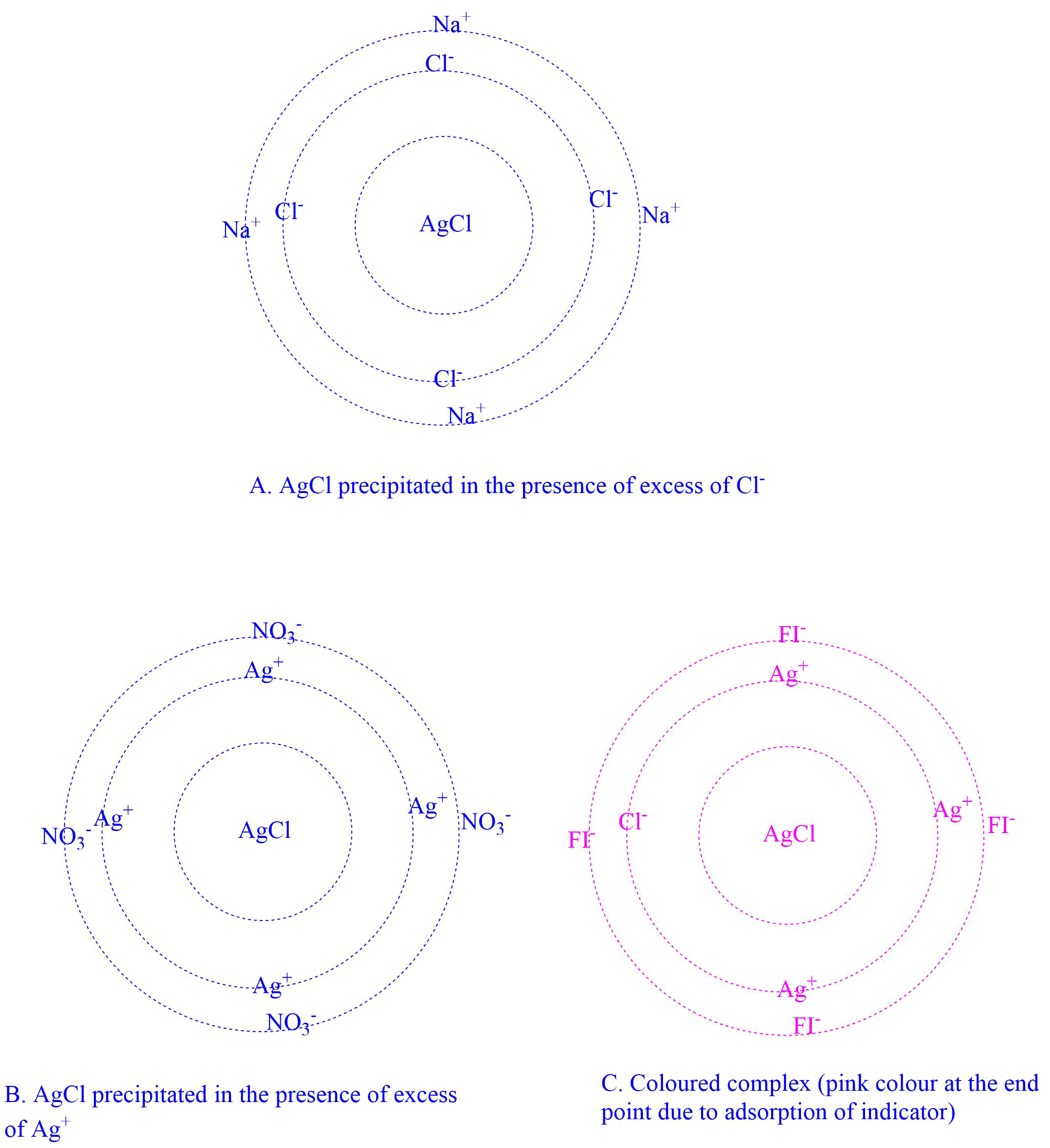
If an indicator, such as fluorescein, is present in the solution, the negative fluorescein ion, which is more strongly adsorbed than the nitrate ion, is immediately adsorbed and will reveal its presence on the precipitation by the formation of a pink complex and a modified flouresceinate ion in the surface.
Condition for the choice of suitable adsorption indicator
The following factors must be considered in choosing the proper adsorption indicator for precipitation titration.
- Coagulation should be prevented as much as possible, and the precipitate should separate ideally in the colloidal form.
- If the solution is excessively dilute, the amount of precipitation formed will be small, and the color change with certain indicators will be mild.
- For the indicator to be mostly in ionic form, the titration solution must have an appropriate pH.
- The indicator ion must have the opposite charge to that of the precipitating agent’s ion The indicator ion must have the opposite charge to that of the precipitating agent’s ion (i.e, titrant).
- The indicator ion must be strongly adsorbed immediately after the equivalence point rather than before the specific compound has entirely precipitated.
Disadvantages of adsorption indicators
One disadvantage of adsorption indicators is that silver halides (AgX) are sensitized to light action by a coating of adsorbed dyestuff. As a result, the titration should be performed with as little exposure to light as possible.
Advantages of precipitation titration
- Precipitation titration is a typical technique for determining the presence of halide ions and certain metal ions in a solution, as well as the salt content of food, beverages, and water.
- It is easy to perform.
- Precipitation titration can provide the rapid and precise result of the analysis.
- For this process, minimum and generally used laboratory apparatus such as burette, pipette, funnel, conical flask, beaker, burette stand, wash bottle are required.
- Fajan method is that it can selectively work with different indicators over different pH ranges.
Disadvantages of precipitation titration
- During the precipitation titration, the equipment must be calibrated properly, otherwise, the outcome will be affected.
- Precipitation titration methods are limited mainly to those in which silver ions are precipitated with anions such as halogens or thiocyanate (SCN-).
- It is hard to achieve precipitate at very dilute solution.
- The Mohr method only works at pH levels between 7 and 10, and it is incompatible with iodide.
Sy
References
- https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Titration/Precipitation_Titration.
- https://www.vedantu.com/chemistry/precipitation-titration.
- https://faculty.ksu.edu.sa/sites/default/files/unit_13_-precipitation_titration_-_subjects_2_0.pdf.
- https://uomustansiriyah.edu.iq/media/lectures/4/4_2019_04_11!10_46_51_AM.pdf
- https://webstor.srmist.edu.in/web_assets/srm_mainsite/files/downloads/Precipitation_Titration.pdf.
