Potassium is the first element of the fourth row in the periodic table. It is denoted by the symbol ‘K’. It is the nineteenth (19th) on the periodic table of elements, and is seventh (7th) most abundant element on Earth’s crust. It’s a silvery-white in color that’s soft enough to cut with a knife. It is an alkali metal which can be found naturally as mineral (usually igneous) and salt compounds.
It was first isolated from potash, the ashes of plants, from which its name derives. Potassium ions are vital for the functioning of all living cells.
History of Potassium
In the year 1807, Sir Humphry Davy firstly isolated metallic potassium through the electrolysis of molten caustic potash (KOH).
The element potassium gets its name from the term ‘potash’, which suggests to an early method of obtaining different potassium salts: putting ash from burned wood or tree leaves in a pot, adding water, heating, and draining the solution.
Martin Klaproth, a German scientist, identified “potash” in the rocks leucite and lepidolite in 1797, and understood that “potash” was not a byproduct of plant development, but rather contained a new element, which he recommended naming kali.
The letter K arose from ‘Kali’ the root word alkali, which is derived from the Arabic word al-qalyah, which means ‘plant ashes’.
Occurrence of Potassium
- Although potassium is the eighth most abundant element on earth and comprises about 2.1% of the earth’s crust, it is a very reactive element and is never found free in nature.
- Most potassium minerals are insoluble and the metal is obtained from them only with great difficulty.
- Potassium is also found in the ocean, but is present only in relatively small amounts, compared to sodium.
- Most potassium occurs in the Earth’s crust as minerals, such as feldspars and clays. Potassium is leached from these by weathering, which explains why there is quite a lot of this element in the sea (0.75 g/liter).
Minerals mined for their potassium are pinkish and sylvite, carnallite and alunite. - The waste liquors from certain saltworks may contain up to 40 grams per litre of potassium chloride and are used as a source of potassium.
- Today, the majority of potassium minerals originate from Canada, the United States, and Chile. The global output of potassium ores is around 50 million tonnes, with large reserves (more than 10 billion tonnes).
Potassium is an essential plant element. Although it is water soluble, little is lost from undisturbed soils because, when it is released from dead plants and animal excrements, it immediately becomes tightly linked to clay particles and is kept ready to be reabsorbed by the roots of subsequent plants.
Most commercial potassium compounds (often loosely called potash) are obtained via electrolysis from soluble potassium compounds, such as:
- Carnallite (KMgCl3∙6H2O)
- Sylvite (potassium chloride, KCl)
- Polyhalite (K2Ca2Mg[SO4]4∙2H2O)
- Langbeinite (K2Mg2[SO4]3)
Isotopes of Potassium
Although potassium has just two stable isotopes (K-39 and K-41), the long-lived radioisotope K-40 is also considered stable. Potassium isotopes, namely K-40 and K-41, are utilized to investigate the effects of potassium on plant development and the human cardiovascular system.
Potassium has three isotopes found in nature:
| Symbol | Natural Abundance |
|---|---|
| 39K | 93.26% |
| 40K | 0.01% |
| 41K | 6.73% |
- Potassium-39 is normally about 13.5 times more plentiful than potassium-41.
- Potassium-37 isotopes might be utilized to directly test the weak interaction in the conventional model. Because the weak interaction is responsible for radioactive decay, the half-lives of specific isotopes may be used to assess the standard model’s description of the interaction.
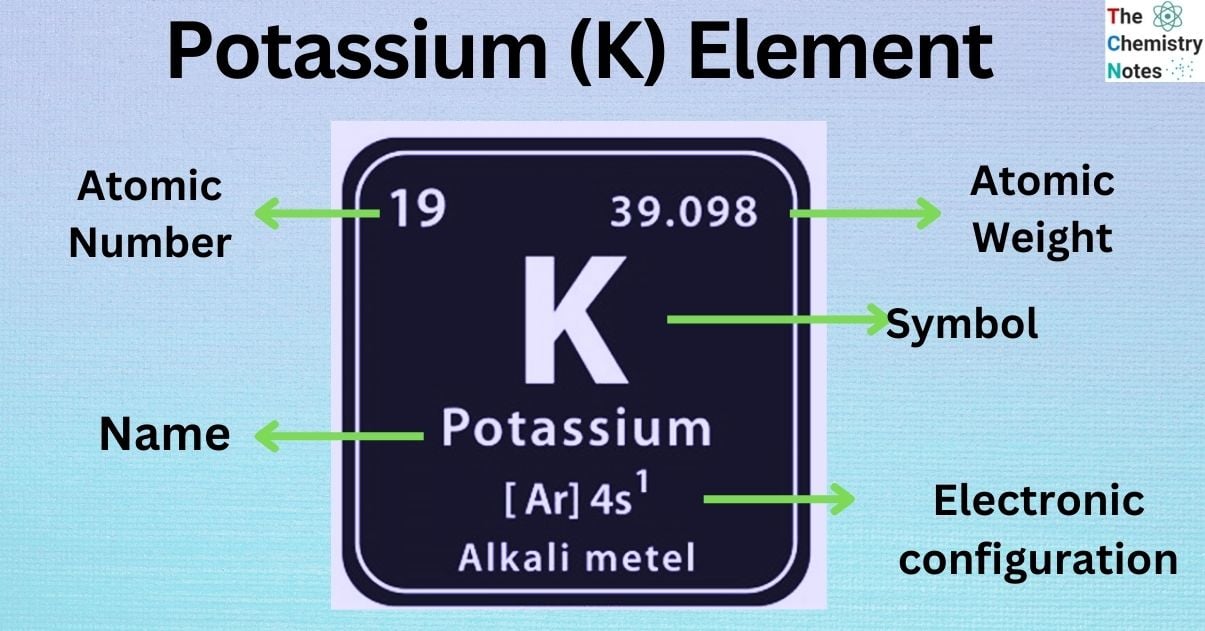
Elemental Properties of Potassium
| Electronic Configuration | [Ar] 4s1 |
| Atomic Number | 19 |
| Atomic Weight | 39.0983 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 1, 4, s-block |
| Density | 0.89 g.cm -3 at 20 °C |
| ionic radius | 0.133nm |
| Van der Waals radius | 0.235 nm |
| Electron shells | 2, 8, 8, 1 |
| Electrons | 19 |
| Protons | 19 |
| Neutrons in most abundant isotope | 39K |
Physical Properties of Phosphorus
- It is a soft and shiny metal which has a melting point of 63 degrees celcius and a boiling point of 770 degrees.
- Potassium metal is soft and white with a silvery luster, has a low melting point, and is an excellent heat and electrical conductor.
- Potassium metal has the ability to float in water.
- Potassium is the second least dense metal after lithium.
- Potassium seems silvery, but when exposed to air, it tends to tarnish toward gray immediately.
- In a flame test, potassium and its compounds emit a lilac color .
| Color/physical appearance | White with Silvery luster |
| Melting point/freezing point | 63.5°C, 146.3°F, 336.7 K |
| Boiling point | 759°C, 1398°F, 1032 K |
| Density | 0.89 (g cm−3) |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 0.82 |
Chemical Properties of Potassium
Like the other alkali metals, potassium is very active.
- Potassium reacts with oxygen, water, and carbon dioxide components in the air.
- When exposed to oxygen, it forms potassium peroxide.
- If potassium is exposed with water it forms potassium hydroxide (KOH). It can react with water violently to produce hydrogen (H2) gas. The excessive heat generated by this reaction, together with the H2 as a product, can be explosive.
- When potassium is burnt in the air, it can produce the orange potassium superoxide KO2.
- Potassium can also react rapidly with all the halogens (F2, Cl2, I2) to form potassium halides (KF, KCl, KBr).
Chemical Reaction of Potassium
Reaction of Potassium with air
Potassium is a soft metal that may be effortlessly sliced. The finished surface is bright and lustrous. This surface, however, quickly tarnishes because to the contact with oxygen and moisture in the air. When potassium is burnt in air, it mostly produces orange potassium superoxide, KO2.
K(s) + O2(g) → KO2(s)
Reaction of Potassium with water
Potassium metal interacts with water relatively quickly to generate a colorless solution of potassium hydroxide (KOH) and hydrogen gas (H2). Because of the dissolved hydroxide, the resultant solution is basic. This reaction is exothermic. The potassium metal becomes so heated early in the reaction that it catches fire and burns with a distinctive pale lilac color.
2K(s) + 2H2O → 2KOH(aq) + H2(g)
Reaction of Potassium with the halogens
To generate potassium halides, potassium metal interacts aggressively with all halogens. As a result, it interacts with fluorine, F2, chlorine, Cl2, bromine, I2, and iodine, I2 to generate potassium(I) bromide, KF, potassium(I) chloride, KCl, potassium(I) bromide, KBr, and potassium(I) iodide, KI, respectively.
2K(s) + F2(g) → KF(s)
2K(s) + Cl2(g) → KCl(s)
2K(s) + Br2(g) → KBr(s)
2K(s) + I2(g) → KI(s)
Reaction of Potassium with acids
Potassium metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated K(I) ion together with hydrogen gas, H2
2K(s) + H2SO4(aq) → 2K+(aq) + SO42-(aq) + H2(g)
Reaction of Potassium with bases
Potassium metal interacts with water extremely quickly to generate a colorless basic solution of potassium hydroxide (KOH) and hydrogen gas (H2). Even after the solution becomes basic, the reaction continues. Because of the dissolved hydroxide, the resultant solution is basic. Exothermic is the response. The potassium metal becomes so heated early in the reaction that it catches fire and burns with a distinctive pale lilac color.
2K(s) + 2H2O → 2KOH(aq) + H2(g)
Uses of Phosphorus
Potassium is regarded as one of the most crucial elements for both the planet and the human body. The loss of potassium from our earth or bodies can cause a variety of problems and hazards. It has a variety of applications that are critical to the smooth operation of our daily lives, but it must be treated with caution because it is a highly reactive element. Potassium has several applications in our daily lives as well as in industrial processes.
Use in medicine
- Potassium citrate is used to treat renal tubular acidosis, a kind of kidney stone.
- Potassium chloride, in the form of medicine, is used for both prevention and treatment of low blood potassium. Low blood potassium levels can develop as a result of diarrhea, vomiting, or some drugs.
Uses as Fertilizer
The greatest demand for potassium compounds is in fertilizers. Potassium is an essential nutrient for plants. This element regulates the operation of stomata in plant cells as well as numerous plant enzymes. It allows the stomata to properly control water inside the plant and reduces water loss through it. Plants are sensitive to illnesses and unable to tolerate heat stress in the lack of potassium. If the soil is lacking in potassium, potassium nitrate is utilized as a fertilizer.
Uses in the Human Body
Potassium is an essential mineral for our body. It is essential to the body’s basic functioning. It aids in the maintenance of acidity and blood pressure. It also regulates water balance, muscular contraction (particularly that of the cardiac muscles), protein metabolism, and other processes. It is critical that human potassium levels are appropriately balanced.
Industrial Uses
- Potassium superoxide (KO2) can create oxygen from water vapor (H2O) and carbon dioxide (CO2) through the following reaction:
2KO2 + H2O + 2CO2 → 2KHCO3 + O2
- It is used in respiratory equipment and is produced by burning potassium metal in dry air.
- Potassium carbonate is used in the manufacture of glass.
- Potassium hydroxide is used to make detergent and liquid soap.
- Potassium chromate (K2CrO4) is a chemical that is widely used in the production of numerous everyday products like Inks, colors, safety matches, fireworks, fly paper, and many more other materials. It is also required in the tanning of leather.
- Potassium cyanide has the ability to dissolve precious metals such as gold and silver. As a result, it has a significant industrial use in gold mining.
- Potassium forms an alloy with sodium (NaK) that is used as a heat transfer medium in some types of nuclear reactors.
Health Effects of Potassium
- Potassium can also be found in fruits and vegetables. Banana, potatoes, meat, bread, milk, and nuts are some of the food source of potassium.
- It aids nerve functioning and has a crucial part in the physical fluid system of humans.
- Potassium, as the ion K+, accumulates inside cells, where it accounts for 95% of the body’s potassium. When our kidneys fail, there will be a buildup of potassium. This can result in irregular heartbeats.
- When you breathe in potassium, it might have an effect on you. Inhaling dust or mists can cause irritation of the eyes, nose, throat, and lungs, resulting in sneezing, coughing, and sore throat.
- Higher levels of exposure may result in a buildup of fluid in the lungs, which can lead to mortality.
- Skin and eye contact can result in serious burns and lasting damage.
- Too much potassium in the blood would cause low blood pressure or even a heart attack.
Environmental Effects of Potassium
There are no such known environmental effects of potassium till date. Even though it may not have an adverse effect on environment, potassium must be handled safely during experiment due to its highly reactive characteristics.
Biological Importance of Potassium
Potassium (K) is the most prevalent inorganic cation and is essential for plant development. K is an activator of several essential enzymes, including those involved in protein synthesis, sugar transport, N and C metabolism, and photosynthesis. K is also essential for cell proliferation, which is essential for plant function and development
- Potassium ions are mostly present inside cells. A 70 kg individual possesses around 90 g of sodium and 170 g of potassium, but only 5 g of iron and 0.06 g of copper. Potassium ions are more prevalent inside cells than sodium ions, and they may also be detected in blood plasma outside the cell.
- The osmolarity (the concentration of a solution represented as the total number of solute particles per liter) of the cell is maintained by potassium ions. They also control the stomatal opening and shutting.
- It controls the heartbeat, promotes normal muscle and nerve function, and is essential for protein synthesis and glucose metabolism.
- Potassium ions are cofactors for enzymes like pyruvate kinase.
- Potassium is essential for heart function, as well as bone and muscle contraction.
- In plant tissue, potassium is related with the flow of water, minerals, and carbohydrates. It is involved in enzyme activation within the plant, which impacts the creation of protein, starch, and adenosine triphosphate (ATP). The rate of photosynthesis may be controlled by the creation of ATP.
Fun Facts About Potassium
- Pure potassium usually is stored under oil or kerosene because it oxidizes so readily in air and reacts in water to evolve hydrogen, which may be ignited from the heat of the reaction.
- The vast majority of potassium atoms in the cosmos were created in the last moments of massive stars as they erupted in supernovae. When stars burst, their oxygen-burning shells produce potassium.
- Potassium is utilized as a heat transfer medium. Its salts are employed as a fertilizer, oxidant, colorant, to produce strong bases, as a salt alternative, and in a variety of other purposes. Potassium cobalt nitrite is a yellow pigment known as Cobalt Yellow or Aureolin.
Learn something interesting about Potassium. Watch out for the video.
References
- https://www.britannica.com/science/potassium
- Burkhardt, Elizabeth R. (2006). “Potassium and Potassium Alloys”. Ullmann’s Encyclopedia of Industrial Chemistry. Vol. A22. pp. 31–38. doi:10.1002/14356007.a22_031.pub2. ISBN 978-3-527-30673-2.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- McNaught, A. D. and Wilkinson,A. eds. (1997). Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). IUPAC. Blackwell Scientific Publications, Oxford.
- Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2007). “Potassium”. Lehrbuch der Anorganischen Chemie
- https://www.lenntech.com/periodic/elements/k.htm
- https://chemistrytalk.org/potassium-element/
- https://byjus.com/chemistry/potassium/
- Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings: https://doi.org/10.3389/fpls.2020.00904

