Polymer is composed of two Greek words (i.e., poly= many and mer= simple unit). So, polymers are large molecules (macromolecules) formed by the combination of simple units. The simple compounds from which polymers are formed are called monomers.
| Name of monomer | Name of polymer |
| Ethene | Polythene |
| Tetrafluoro ethylene | Teflon |
| Ethyl Acrylate | Polyethyl Acrylate |
| Chloroprene | Neoprene |
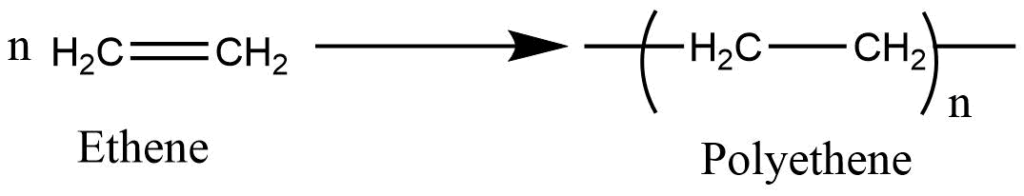
What is Polymerization?
The process by which the monomer molecules are linked to form a large polymer molecule with or without the elimination of simple molecules like water, HCl, NH3, etc.
Classification based on the mode of polymerization:
- Addition polymerization
- Condensation polymerization
Addition polymerization:
It involves the chain addition of the monomers containing double bonds to form the macromolecules having high molecular weight i.e. polymers. This proves involves without the elimination of small molecules like water, HCl, and NH3.
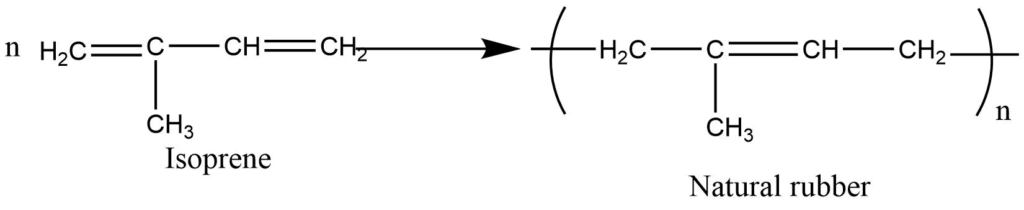
Natural rubber contains isoprene (2-methyl-1,3-butadiene) repeat unit.
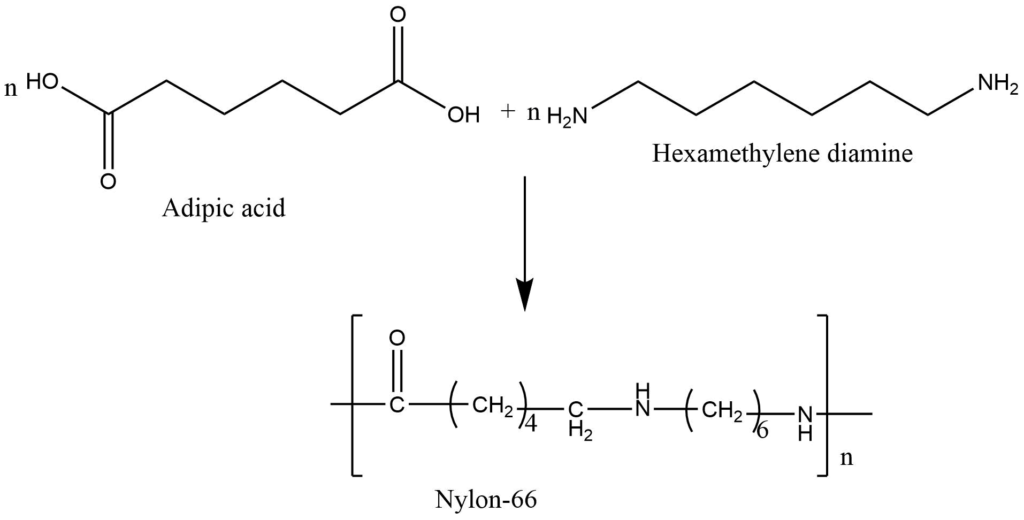
Condensation polymerization:
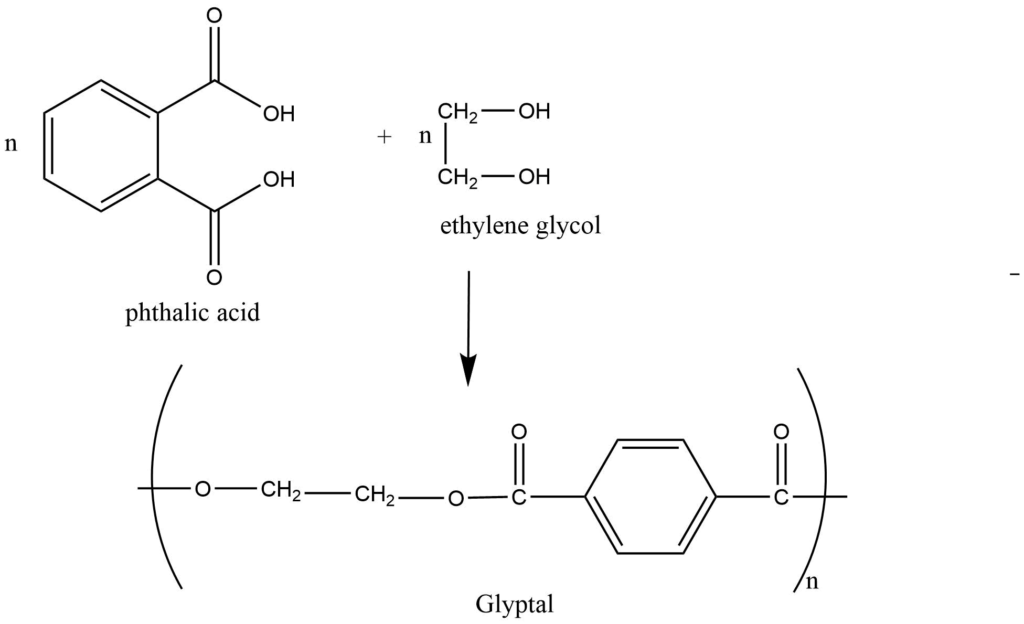
This is the process in which, two or bi-functional monomers undergoes a series of condensation reaction with the simultaneous loss of small molecules like H2O, NH3, and HCl to form the polymers. The polymers so formed are called condensation polymers.Formation of nylon-66: Nylon-66 is obtained by condensation polymerization of adipic acid and hexamethylenediamine
Formation of peptides (natural polymers): Proteins are condensation polymers of different amino acids that have been condensed in a specific sequence by the loss of water molecules.
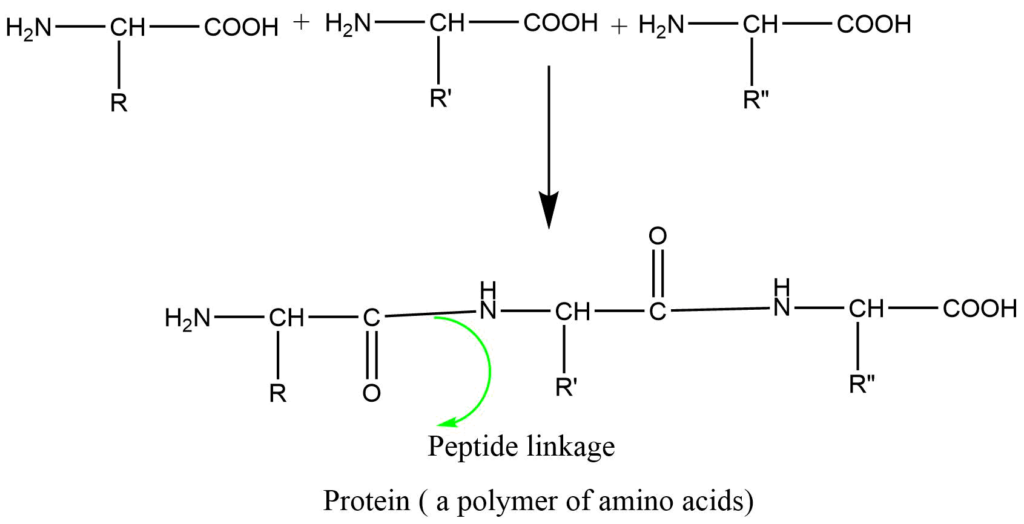
Classification of polymers on the basis of their origin:
Natural polymers:
The polymers which occur naturally, either in plants or animals, are called natural polymers. For example, natural rubber, protein, cellulose, starch
| Natural polymer | Constituent unit | Occurrence |
| Starch | Polymer of glucose | Main food reserve of plants |
| Cellulose | Polymer of glucose | Main structural material of plants |
| Natural rubber | Polymer of isoprene | Occurs as latex in the bark of many tropical trees. |
Synthetic polymers:
They are man-made commercial polymers that are made to have specific properties that cannot be achieved by natural polymers. Synthetic polymers are made into sheets, films, threads, and fibers and are also designed to have special mechanical properties. They can withstand high temperatures. Eg. Nylon Bakelite
Semisynthetic polymers:
Apart from the synthetic and natural polymers, there is another type of polymer called semi-synthetic polymer, which is formed by the modification of the natural polymer. Eg. Nitrocellulose, cellulose acetate
Classification of polymers on the basis of type of monomers:
Homopolymers:
Homopolymers are polymers obtained from identical monomer units. E.g. polythene, polyvinyl chloride, etc.
Copolymers:
Copolymers are polymers obtained from two different monomeric units. E.g Nylon-66, Buna-s rubber
Classification of polymers on the basis of the structures
Linear polymers:
In linear polymers, monomeric units are linked together to form the linear chain. Various linear chains are stacked over one another to give a well-packed complex structure. The linear polymers have high molecular weight and high tensile strength. They are soluble in organic solvents, so many of them are used to make paints and lacquers. Common examples of linear polymers are polythene and polyesters.
Branched-chain polymers:
The branched-chain polymers are polymers made up of linear chains containing a small chain as the branch of the main chain. They don’t have a compact structure. They usually have lower melting points and densities as compared to linear chain polymers. E.g. glycogen, amylopectin, etc.
Cross-linked polymers (three-dimensional polymers):
In this polymer, the monomeric units are arranged in a three-dimensional network. Cross-linked polymers are insoluble in any kind of solvent. They are chemically inert.
Classification of polymers based on the molecular forces:
The practical utility of polymers depends on their mechanical properties like elasticity, toughness, and tensile strength. The mechanical properties of the polymers depend on the strength of forces acting between the chains. So the polymers can be classified into the following categories:
Elastomers:
Elastomers are made up of polymeric chains that are held together by weak intermolecular forces. The weak intermolecular force makes them able to stretch by applying the forces. The elastomers become soft on heating and can be molded into different shapes.
Fibrous polymers:
These polymers have strong intermolecular forces in the form of hydrogen bonds and dipole-dipole interaction. They are thin thread-like crystalline structures having high tensile strength and low elasticity. E.g Dacron, Orlon, Nylon-66.
Thermosetting polymers:
Thermosetting polymers are formed by heating relatively low molecular weight semi-fluid polymers. They become infusible and form a hard mass on heating due to cross-linking. They cannot be reshaped by heating. e.g., phenol-formaldehyde and melamine formaldehyde resign are examples of thermosetting plastics.
Classification based on the sequence of the synthesis
Chain growth polymerization:
In chain-growth polymerization, the monomer is added to the growing chain one at a time. The chain growth polymerization requires the initiators like organic Pero-oxides e.g benzoyl peroxides and t-butyl peroxides. These initiators decompose by heating, oxidation, or by reduction to produce free radicals, carbocations, or carbanions.
Chain growth polymerization proceeds by one of the following mechanisms
- Free radical polymerization
- Cationic polymerization
- Anionic polymerization
Each mechanism has three steps:
- Initiation step: Strat the polymerization process
- Propagation step: Allows chain to grow
- Termination step: Stop the chain from growing.
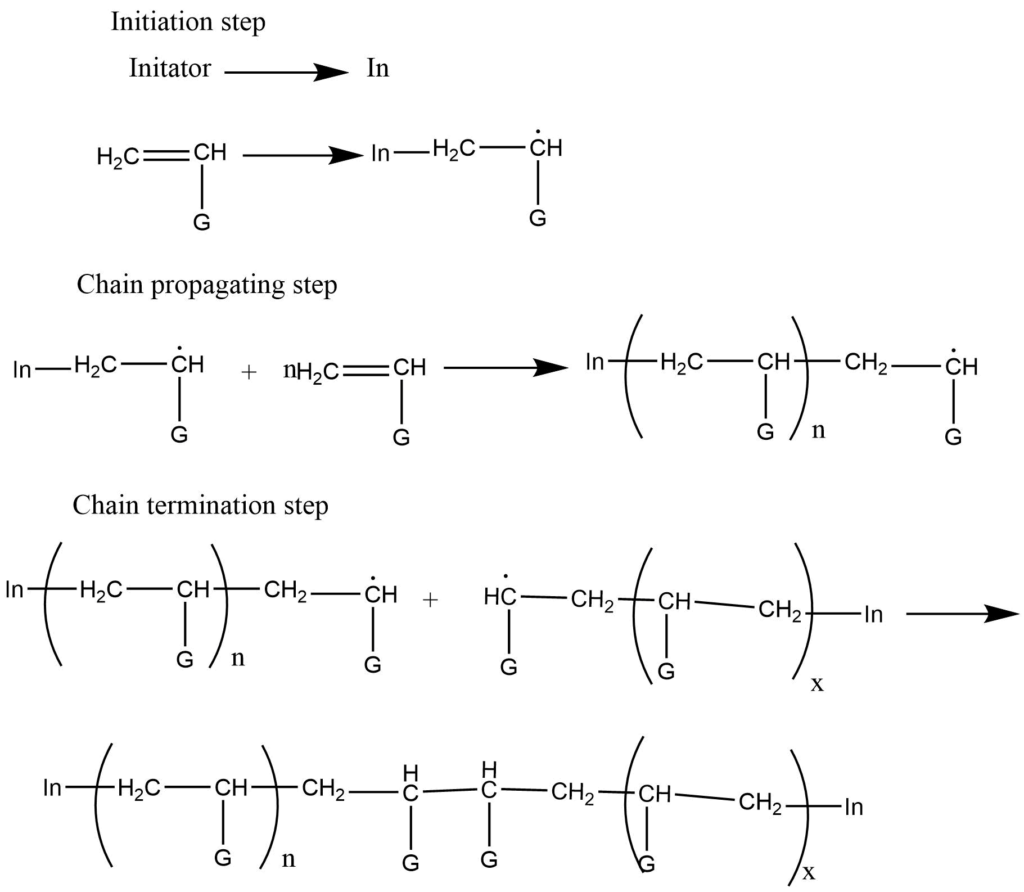
- Free radical polymerization: It takes place in presence of the free radical producing initiators. E.g t-butyl peroxide.
The initiator (In) first adds to the monomer to form a new radical of large size. The repeated addition of thus formed new radicals with other monomers leads to the formation of a bigger free radical. At last, these radicals react themselves, and chain termination occurs.
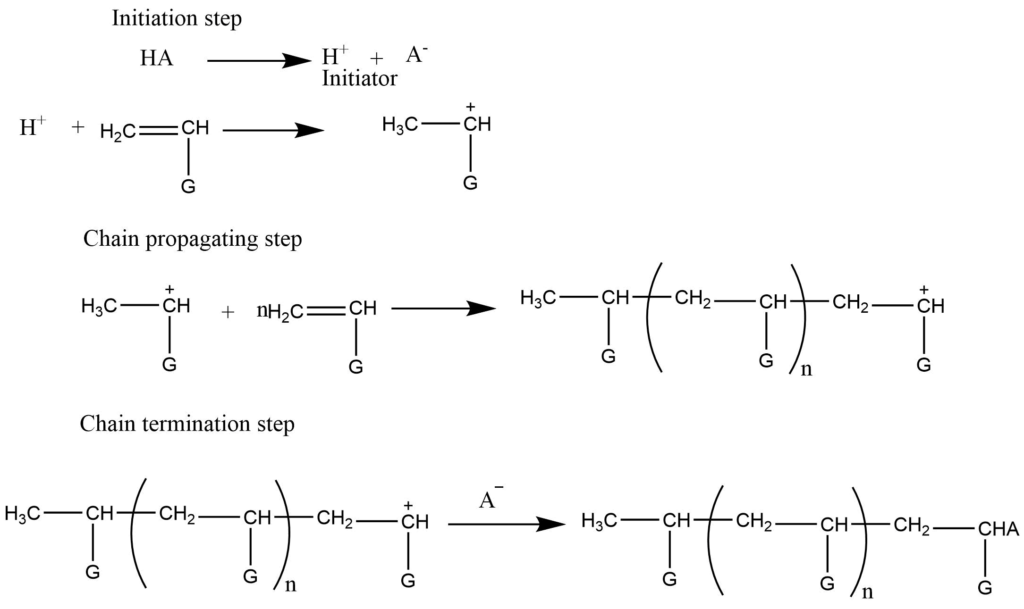
- Cationic addition polymerization: Cationic polymerization involves cation as an initiator which adds to the monomers to provide cationic intermediates. This [process is initiated by the acid which provides H+ ions. Electron donating groups generally facilitate cationic polymerization. The electron releasing group like the alkyl group, stabilized the intermediate cation by hyperconjugation while the other group stabilized the intermediate by resonance.
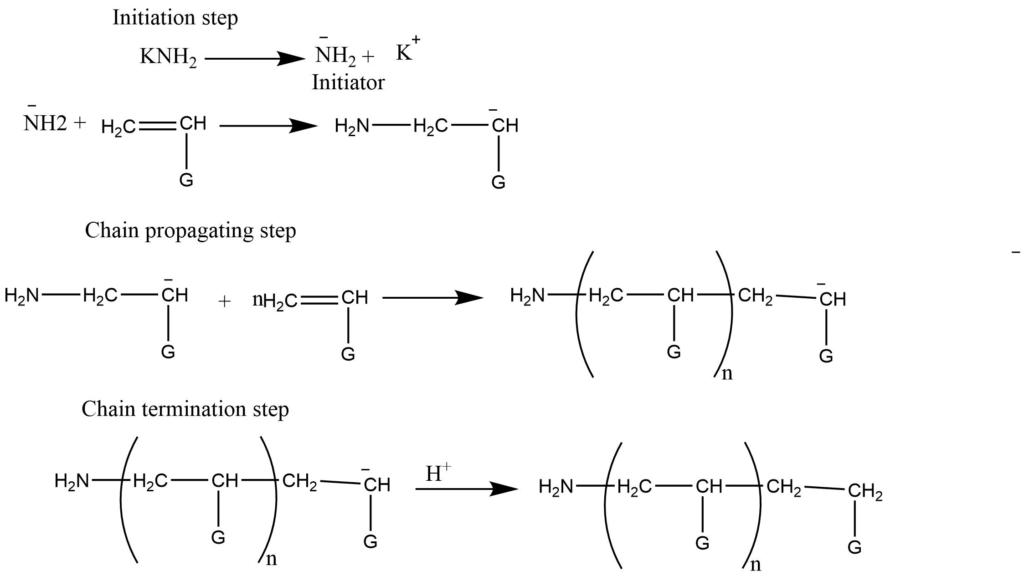
- Anionic addition polymerization: In this process anions (like NH2– formed by KNH2) add to the monomers to form the anionic intermediates, which in further addition to monomers undergo chain growth. This process is terminated by adding acids that provide H+ ions. Usually, the electron-withdrawing groups like -C6H5, NO2 facilitates anionic addition polymerization.
Step growth polymerisation:
Step growth polymerization involves the condensation between polyfunctional monomers in a stepwise manner with or without the elimination of small molecules like water, ammonia, and so on.
Uses of polymers
- Polymeric materials are used to form plastic bags, bottles, lab wares, and also for electronic uses.
- Biopolymers are used for the replacement of heart valves and blood vessels.
- They are also used in contact lenses.
- They are used in Automobile parts as wood substitutes.
- Polymers are also used as insulators.
- polymeric films are used to cure wounds caused by burns.
References
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/polymers.htm
- https://whatispiping.com/polymers-types-examples/
- https://byjus.com/jee/polymers/
- https://byjus.com/questions/define-the-term-polymerisation/
- https://study.com/learn/lesson/polymerization-overview-process-examples.html
- https://byjus.com/questions/glyptal-is/
- coursesonline.iasri.res.in/mod/page/view.php?id=997
- https://www.bbc.co.uk/bitesize/guides/zyfgmnb/revision/4
