Polar Molecules Definition
A polar molecule is a chemical substance in which the distribution of electrons between the atoms involved is uneven, resulting in a dipole moment.
- The term polarity is used to describe the difference in electrical poles of a molecule which then determines how polar the molecule is.
- The determination of whether or not a molecule is polar comes from the sum of all the bonds present in the molecule.
- Polarity is developed when a bond is formed with atoms having a difference in their electronegativities. When one of the atoms has a higher electron affinity, it attracts the shared pair of electrons towards it, which changes the distribution of electrons.
- If the difference in electronegativity is considerable, a polar bond with form. If the difference is extreme, one of the atoms completely strips the electrons towards it, resulting in an ionic bond.
- Polarity is often expressed in the case of covalent bonds, as these bonds are formed by the sharing of electrons.
- Polar molecules orient themselves in a particular direction in the presence of an electric field. The positive end of the molecules directs towards the negative pole of the field, whereas the negative end directs towards the positive pole.
- In the case of molecules with more than two atoms, the geometry of the molecules should be taken into account in order to determine their polarity.
- In polyatomic compounds, polarity depends on the net dipole moment of all the covalent bonds present in the molecule.
- Polar molecules usually have a higher boiling and melting point as well as a high surface tension as polar linkages are considerably stronger than nonpolar linkages.
- Some examples of polar molecules are H2O, CHF3, NH3, etc.
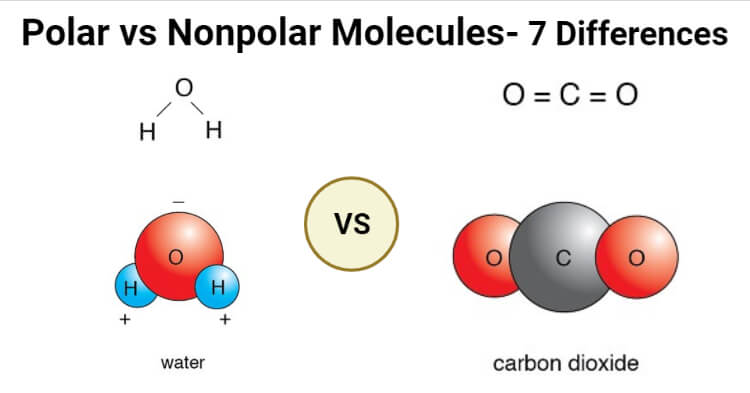
Image Source: University of Hawaiʻi at Mānoa.
Nonpolar Molecules Definition
Nonpolar molecules are the molecules in which the electrons are equally shared among the involved atoms and have a zero dipole moment.
- Nonpolar molecules are formed when the two atoms involved in a chemical bond have a similar affinity towards electrons which enables equal distribution of electrons between them.
- True nonpolar bonds exist only between diatomic molecules of the same element like N2 and O2.
- Nonpolar molecules do, however, exhibit London dispersion forces induced as a result of the symmetrical distribution of electrons.
- The interaction is often spontaneous and temporary and is the weakest of all intermolecular forces.
- In the case of polyatomic or complex molecules, the lack of polarity can be a result of the symmetrical arrangement of polar bonds.
- If two equally polar bonds are arranged in a way that the dipole of one bond cancels out the dipole of the other, a zero net dipole can be generated, resulting in a nonpolar molecule.
- Most hydrocarbons are nonpolar in nature, as the difference in electronegativity between carbon and hydrogen atoms is very small.
- The nonpolar interactions are usually less strong than polar interactions as no electrostatic association is involved.
- Nonpolar molecules do not show any orientation in an electric field as no electrostatic interactions occur between nonpolar molecules and the electric field.
- Some examples of nonpolar molecules are CO2, H2, benzene, etc.
7 Major Differences (Polar vs Nonpolar Molecules)
| Characteristics | Polar Molecules | Nonpolar Molecules |
| Definition | A polar molecule is a chemical substance in which the distribution of electrons between the atoms involved is uneven, resulting in a dipole moment. | Nonpolar molecules are the molecules in which the electrons are equally shared among the involved atoms and have a zero dipole moment. |
| Dipole moment | A net dipole moment is present in polar molecules. | The net dipole of nonpolar molecules is zero. |
| Electronegativities | The difference in electronegativity between the atoms is greater than 0.4. | The difference in electronegativity between the atoms is less than 0.4. |
| Physical properties | Polar molecules have high boiling and melting points. | Nonpolar molecules have low boiling and melting points. |
| Charge separation | Polar molecules have partial positive and partial negative charges within the molecule. | No charge separation can be observed in the case of nonpolar molecules. |
| Intermocleular forces | Polar molecules might be involved in hydrogen bonding between the charged poles of the bond. | Nonpolar molecules usually have weaker intermolecular forces like van der Waal’s forces. |
| Examples | Some examples of polar molecules are H2O, CHF3, NH3, etc. | Some examples of nonpolar molecules are CO2, H2, benzene, etc. |
Examples of Polar Molecules

Image Source: University of Hawaiʻi at Mānoa.
Water (H2O)
- Water is a polar molecule composed of two O-H bonds where the dipole moment of each of those bonds is equal to 1.5D.
- Water doesn’t have a linear structure as the lone pair of electrons on the oxygen atom repels the shared paired of electrons between hydrogen and oxygen.
- The oxygen atom is more electronegative than hydrogen, and thus, it attracts the shared pair of electrons towards itself.
- The unequal distribution of electrons results in a partial negative charge on the oxygen atoms and a partial positive charge on the hydrogen. The polarity of the O-H bonds is responsible for the bent structure of the molecule.
- The polar nature of hydrogen molecules also results in the formation of hydrogen bonds with another water molecule or other polar molecules.
- Water is called a universal solvent as it can dissolve most polar substances as a result of interaction between the slightly charged atoms.
Examples of Nonpolar Molecules
Image Source: University of Hawaiʻi at Mānoa.
Carbon dioxide (CO2)
- Carbon dioxide is composed of two C-O polar bonds; however, the molecule is nonpolar as the bonds are arranged in a way that the net dipole is zero.
- Each of the C-O bonds has a dipole moment of 2.3D, but the dipoles act in opposite directions, resulting in the net-zero dipole.
- The C-O bonds are arranged at 180° relative to one another, resulting in a linear molecular structure.
- The polarity of the C-O is due to the fact that the oxygen atom is more electronegative than the carbon atom.
- Carbon dioxide is an example of molecules that are nonpolar even though the bonds present in the molecule are polar.
FAQs
Is CO2 polar or nonpolar?
CO2 (Carbon dioxide) is a nonpolar molecule.
Is H2O polar or nonpolar?
H2O (Water) is a polar molecule.
Is NH3 polar or nonpolar?
NH3 is a polar molecule.
Is SO2 polar or nonpolar?
SO2 (Sulfur dioxide) is a polar molecule.
Is BF3 polar or nonpolar?
BF3 (Boron Trifluoride) is a nonpolar molecule.
Is CCl4 polar or nonpolar?
CCl4 (Carbon Tetrachloride) is a nonpolar molecule.
Is CH4 polar or nonpolar?
CH4 (Methane) is a nonpolar molecule.
Is CH2Cl2 polar or nonpolar?
CH2Cl2 (Dichloromethane) is a polar molecule.
Is CH3Cl polar or nonpolar?
CH3Cl (Chloromethane) is a polar molecule.
Is XeF2 polar or nonpolar?
XeF2 (Xenon difluoride) is a nonpolar molecule.
Is NOCl polar or nonpolar?
NOCl (Nitrosyl chloride) is a polar molecule.
Is SF4 polar or nonpolar?
SF4 (Sulfur tetrafluoride) is a polar molecule.
Is BH3 polar or nonpolar?
BH3 (Borane) is a nonpolar molecule.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- https://chem.libretexts.org/Courses/Brevard_College/CHE_103_Principles_of_Chemistry_I/05%3A_Chemical_Bond_II/5.10%3A_Electronegativity_and_Bond_Polarity – 13%
- https://www.vedantu.com/chemistry/polarity – 10%
- https://pediaa.com/difference-between-polar-and-nonpolar-molecules/ – 6%
- https://techiescientist.com/is-pf5-polar-or-nonpolar/ – 3%
- https://www.thoughtco.com/definition-of-polar-molecule-605531 – 2%
- https://courses.lumenlearning.com/cheminter/chapter/polar-molecules/ – 2%
