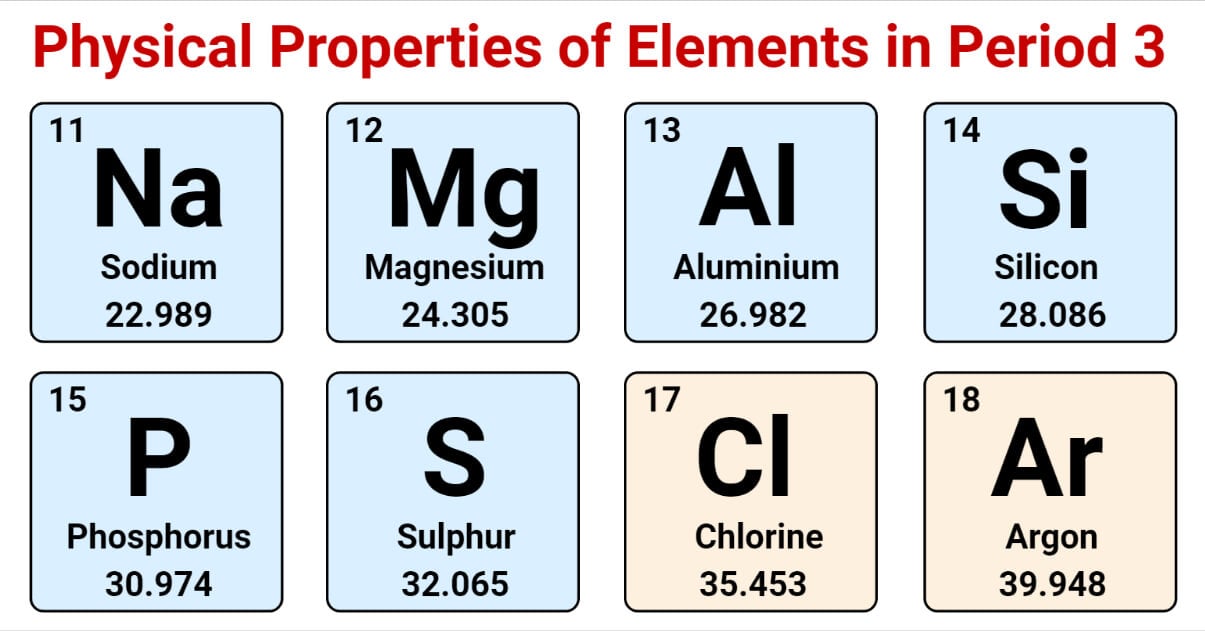
The elements of period 3 are placed in the third row of the periodic table. The elements positioned in period 3 are Sodium (Na), Magnesium (Mg), Aluminium (Al), Silicon (Si), Phosphorus (P), Sulphur (S), Chlorine (Cl), and Argon (Ar).
Firstly, we are well known for the periodicity of the rows in the periodic table. The elements in the periodic table are arranged based on their properties and periodicity. Hence, the elements of period 3 are also related to different physical and chemical properties of each other. Let us further discuss the trends in the properties and comparative study of different elements of period 3 in the periodic table.
Physical Properties of Elements in Period 3
The modern periodic law states- ” The physical and the chemical properties of the elements are the periodic functions of their atomic numbers”. Moreover, electrons, atomic numbers, and molecular structure help us to understand the trends of chemical bonding, chemical properties, and physical properties, respectively.
Physical State of Elements in Period 3
| Elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| Physical State | Solid | Solid | Solid | Solid | Solid | Solid | Gas | Gas |
Atomic Radius of Elements in Period 3
The distance between the nucleus and the outermost electrons of an atom is the atomic radius. But this is just a surface level of understanding because the border of the electron cloud of atoms does not have a definite edge. Their borders are described as the resemblance of the fuzzy cloud, and they are changeable according to the conditions. Therefore, the measurement of the atomic radius is standardized by measuring the distance between the nuclei of two identically bonded atoms and dividing by two. Here’s a pictorial representation.
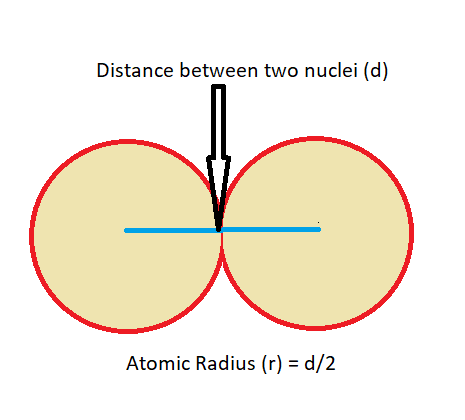
The atomic radius of the elements moving from left to right i.e. ( Na to Ar), decreases. Protons are added to the nucleus as electrons are added to the same principal energy level over time, and due to their increased positive charge, these electrons are gradually drawn closer to the nucleus. The size of the atoms decreases as the force of attraction between nuclei and electrons increases, whereas electron-electron repulsions that would otherwise cause the atom’s size to increase.
Elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| Atomic Radius ( Å) | 1.90 | 1.45 | 1.18 | 1.11 | 0.98 | 0.88 | 0.79 | 0.71 |
Ionic Radius of Elements in Period 3
Although the trend of ionic radius in period 3 is the same as of atomic radius, the measure of the ionic radius is quite uncertain. A positive ion is formed only when one or more electrons are removed from neutral atoms, and a negative ion is formed when additional electrons add up to the neutral atom. Thus, the ionic radius of the element differs with different oxidation states.
| Elements | Na+ | Mg2+ | Al3+ | Si4+ | P3- | S2- | Cl– | Ar |
| Electronic Configuration | 2,8,1 | 2,8,2 | 2,8,3 | 2,8,4 | 2,8,5 | 2,8,6 | 2,8,7 | 2,8,8 |
| Ionic Radius (nm) | 0.102 | 0.072 | 0.054 | – | 0.212 | 0.184 | 0.181 | – |
The relation between the increasing nuclear charge and the decreasing size is concluded based on the study of isoelectronic series i.e., the comparison of the atoms or ions with the same number of electrons but different nuclear charges
Melting Point of Elements in Period 3
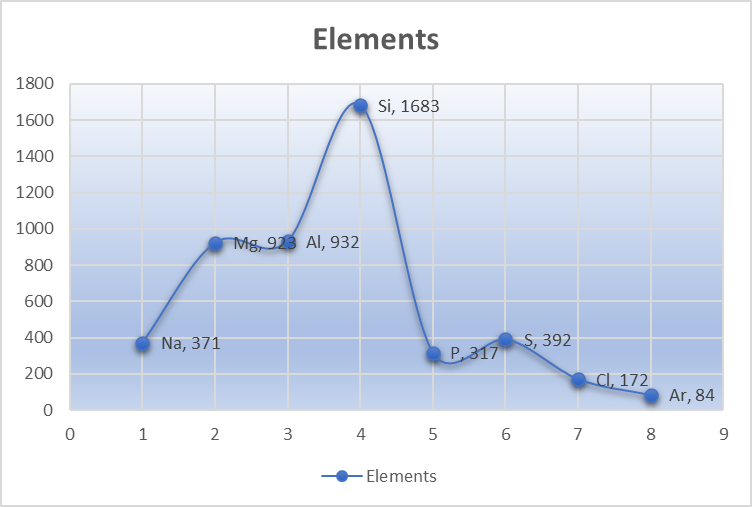
The melting point is defined as the amount of energy required to break a bond(s) and change the phase of the substance from solid to liquid. In the case of period 3 elements, there is a gradual increase in melting point from left to right from Sodium to Silicon and decreases further right with a slight increase from phosphorus to Sulphur.
| Elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| Melting Point (K) | 371 | 923 | 932 | 1683 | 317 | 392 | 172 | 84 |
Above all, silicon has the highest melting point in period 3 because of its three-dimensional covalent structure where all the atoms are bonded with a strong covalent bond. As a result, this requires a large amount of energy.
Electrical Conductivity of Elements in Period 3
Electrical conductivity is the measure of the amount of electric current a material can carry. In other words, the electrical conductivity depends on the presence of free electrons.
Certainly, electrical conductivity and metals come to mind relatively.
Na, Mg, Al: Metals
Si: Metalloid
P, Cl, Ar: Non-metal
In the case of period 3, sodium, magnesium, and aluminum are all metals. Hence, going from Na to Al, the number of delocalized electrons increases, which increases the number of electrons to carry a charge and increase the electric conductivity of elements. Silicon is a semiconductor, and hence electrical conductivity lies in between the metals and non-metals.
P, S, Cl, and Ar are non-metals and hence do not conduct electricity.
| Elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| Electrical Conductivity (S/m) | 21*106 | 23*106 | 38*106 | 1000 | 10-9 | 1.5*10-15 | 0.01 | 0 |
Electronegativity of Elements in Period 3
Electronegativity is the ability of an atom to attract the bonding pairs of electrons. Since Linus Pauling proposed the electronegativity scale, the Pauling scale is used to determine the electronegativity values based on bond energies.
Electronegativity values increases as we move from left to right because the nuclear charge increases towards the right, and subsequently, atomic size decreases. As argon does not form a covalent bond, consequently, no values are assigned.
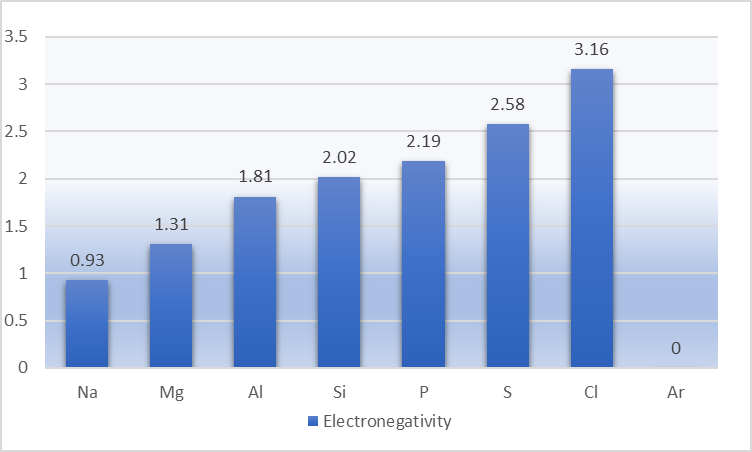
The trend in electronegativity value in elements of period 3 is Na<Mg<Al<Si<P<S<Cl
Electron Affinity of Elements in Period 3
Electron affinity is defined as the energy required to absorb or release energy when an electron is added to a gaseous atom or gaseous ion.
| Elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| Electron Affinity (KJ/mol) | -53 | 230 | -50 | -120 | -74 | -200 | -349 | 35 |
The general trend of electron affinity is to increase moving from the left to right as electrons are added to energy levels, thus increasing the force of attraction. The stronger the attraction more energy is required hence increasing electron affinity. However, the trend doesn’t look in order because of different factors. In the case of group II-A i.e., Mg has positive electron affinity due to their complete s-orbital. Also, in group VIII-A elements, the electron affinity is positive because of filled p-orbitals, and to gain or lose electrons from these groups is harder.
But the second electron affinity of all the elements is positive.
Ionization Energies of Elements in Period 3
Ionization energy (I.E) is defined as the energy required to remove the most loosely bound electron from an isolated gaseous atom or ion and to form a cation.
| Elements | Na | Mg | Al | Si | P | S | Cl | Ar |
| First Ionization Energy (KJ/mol) | 496 | 738 | 578 | 786 | 1012 | 1000 | 1251 | 1521 |
The general trend of ionization energy in the periodic table is it increases with elements moving from left to right because of increasing nuclear charge and decreasing atomic size. There are several anomalies as they do not follow the trend. To clarify, it is difficult to release electrons from half-filled and filled outer electron shells. So, there is high I.E. in elements of group II A and group V A.
There are several factors that affect the ionization energy
- Nuclear charge
- Atomic Size
- Penetrating effect
- Shielding effect
- Electronic configuration
For a quick revision of properties of period 3 elements, you can watch it out.
References
- Smith, D. (1990). Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry (Cambridge Texts in Chemistry and Biochemistry). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511622922
- Mingos, D. M. P. Essential Trends in Inorganic Chemistry. Oxford University Press, 1998.
- Lee, J D. Concise Inorganic Chemistry. London: Blackwell Science, 2006. Print.
- Cotton, F A, and F A. Cotton. Advanced Inorganic Chemistry. , 1999. Print.
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Period/Period_3_Elements/Physical_Properties_of_Period_3_Elements.

