Phosphonitrilic compounds (Phosphazene) are compounds containing a P-N backbone with two substituents on each P atom. Phosphazene is a cyclic and acyclic polymer of phosphorous and nitrogen consisting of a -N=P- units containing P and N atoms with two substituents on each P atom. They are ‘unsaturated PN compounds’ containing phosphorus, mostly in the +V state. They may have cyclic or chain structures as common polymeric structures.
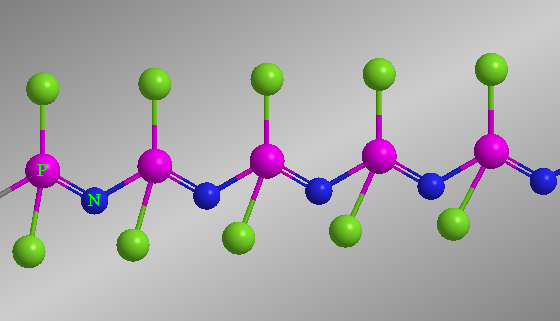
Their water-repellent, solvent-resistant, and flame-resistant properties have led to numerous technological innovations over the last few decades.
Classification based on the number of phosphazenes
They are classified into three groups based on the number of phosphazene units incorporated into the structure.
Monophosphazenes
They are unsaturated compounds of type X3P = NR, (X and R = Cl, OR, NR2, Ar, etc.).
Preparation:
(a) By the reaction of an azide (R – N3) with PR3 (R = Ar, OR, Cl, NR2)
P (C6H5)3 + C6H5–N3 → (C6H5)3 – P = N – C6H5 –N2
(b) Dihalotriphenylphosphenes on reaction with aromatic amines produces monophosphazenes
(C6H5)3PCl2 + C6H5–NH2 → (C6H5)3 – P = N – C6H5 + 2 HCl
![]() Diphosphazene
Diphosphazene
These contain two 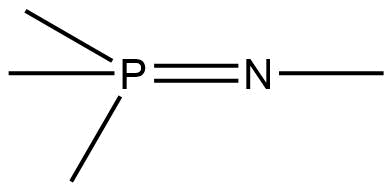 groups and the P = N and the P – N bonds are equivalent. The
groups and the P = N and the P – N bonds are equivalent. The
Preparation
(a) by the reaction of PCl5 with NH4Cl under mild conditions in the presence of chlorohydrocarbon solvents
PCl5 + NH4Cl → [Cl3P = N – PCl2 = N – PCl3] + Cl–
Diphosphazenes exist both in covalent and ionic forms.
Polyphosphazene
These contain more than two  groups.
groups.
1. when PCl5 andNH4Cl are refluxed in an organic solvent like chlorobenzene, tetrachloroethane, a series of phosphonitrilic chlorides of the general formula (PNCl2) n are obtained.
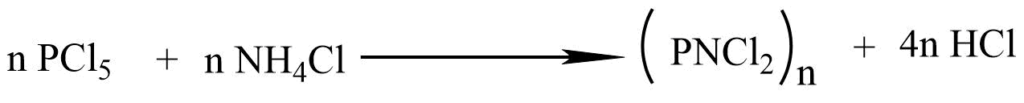
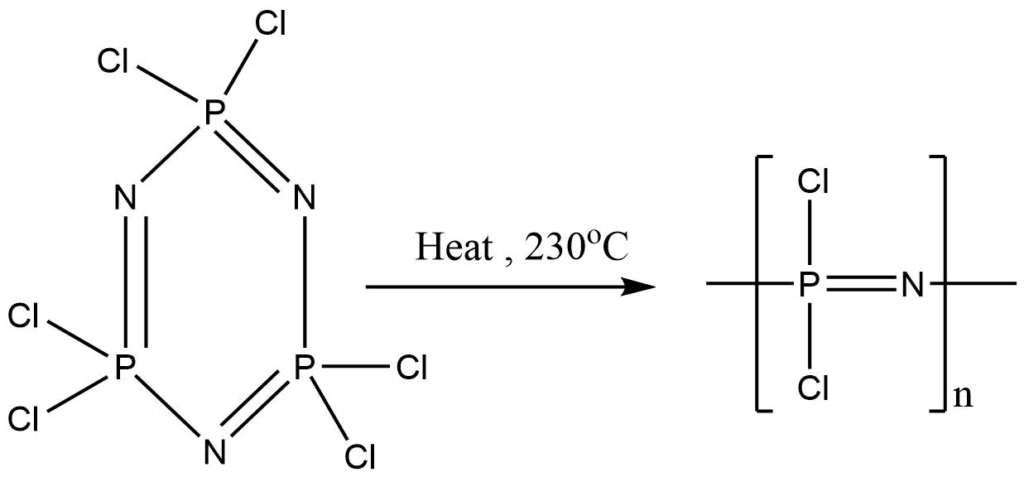
2. (PNCl2) 3 on heating at 230o C, rubber like chain polymer is formed.
Classification of phosphazenes based on the geometric structure.
Acyclic phosphazenes:
They are short-chain linear phosphazenes, also known as phosphine imines or phosphoranimines. They are also known as iminophosphoranes and have attracted attention for various reasons, including their ability to function as strong bases, organocatalysts, and ligands for transition-, lanthanide-, and main-group metal ions.
Cyclophosphazenes
Cyclophosphazenes are the oldest inorganic heterocyclic rings, consisting of a valence unsaturated skeleton with the [N=PR 2] repeat unit. The ring is composed of alternate phosphorus and nitrogen atoms. The phosphorus center is pentavalent and tetracoordinate within these rings, while the nitrogen center is trivalent and di-coordinate.
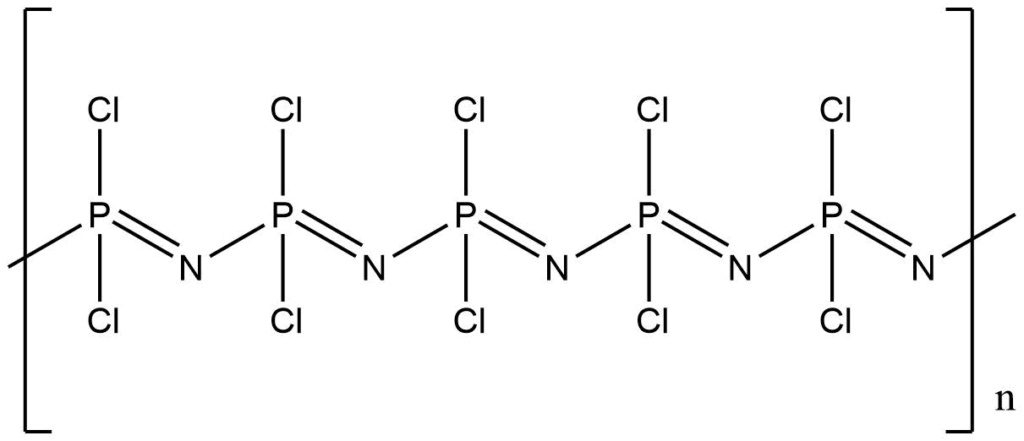
Polyphosphazenes
Polyphosphazenes are hybrid polymers with a flexible inorganic backbone of alternating phosphorus and nitrogen atoms and organic side groups. Their composition varies from 3 to 10,000 (–N=P–) repetitive units having two substituents (–R) attached to the phosphorus atom.
Poly(organophosphazenes) provide an appealing platform for the design and synthesis of novel biodegradable polymers, as well as critical advantages for the design of biologically functional macromolecules with high functional density structural diversity and tailored biodegradability.
Polyphosphazenes possess special properties, including flame-retardant properties, high resistance to oil and solvents, and feasibility for tailored properties according to the choice of organic, inorganic, or organometallic side groups.
Phosphazene Properties
- They have water repellency, solvent resistance, flame resistance, and dielectric properties.
- Phosphonitrilc halide undergoes several substitution reactions in which the halogen atom is replaced by other groups such as OH, OR, NR, NHR, or R to give a fully substituted derivative.
- Phosphonitrilc halide form additional compounds with lewis bases, such as Al2X6 and SbF6.
- X-ray diffraction showed that the trimer tetramers have cyclic, hexagonal, and puckered 8- membered ring structures.
- The P-N distance is nearly equal in these ring systems. They are shorter than the expected single bond distance, which suggests the presence of a double bond between P and N atoms and have a resonance structure.
- The larger polymers are linear in structure.
Uses of phosphazene
- There are many uses for high molecular weight phosphazene as rigid plastic, expanded foam, and fibers.
- Their high thermal stability makes them useable in place of carbon polymer.
- They are used as a slow drug releasing agent.
- They are used for making metal coatings & wire insulation and composite materials together with glass or with ordinary phenolic resins.
- Thin films of poly (aminophosphazenes) are used in hospitals to cover severe burns and other extensive wounds as they prevent the loss of body fluids and keep germs out.
References
- https://www.hindawi.com/journals/ijps/2013/645869/
- https://www.globalspec.com/reference/44298/203279/3-2-cyclophosphazenes
- https://pubs.rsc.org/en/content/chapter/bk9781839162053-00429/978-1-83916-205-3
- http://faratarjome.ir/u/media/shopping_files/store-EN-1429082956-7175.pdf
- https://www.sciencedirect.com/science/article/abs/pii/0010854594800049
- https://polymerdatabase.com/polymer%20classes/Polyphosphazenes.html
