The term “pH” stands for “potential of hydrogen,” and it is a unit of measurement for the concentration of hydrogen ions in a solution. The concept of pH was first introduced by the Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909.
Definition
pH is defined as the negative logarithm of the concentration of H+ ions. As a result, the meaning of the name is justified as hydrogen power.
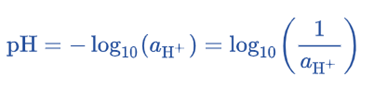
We know that not all acids and bases react at the same rate with the same chemical compound. Some react violently, others moderately, and still others do not. To quantify the strength of acids and bases, we use a universal indicator that changes color depending on the concentration of hydrogen ions in the solution. In general, the value of acids and bases is used to quantify their strength.
Although less intuitive, the mathematical definition is more useful overall. It states that pH equals the negative logarithmic value of hydrogen ion (H+) concentration.
pH = -log [H+]
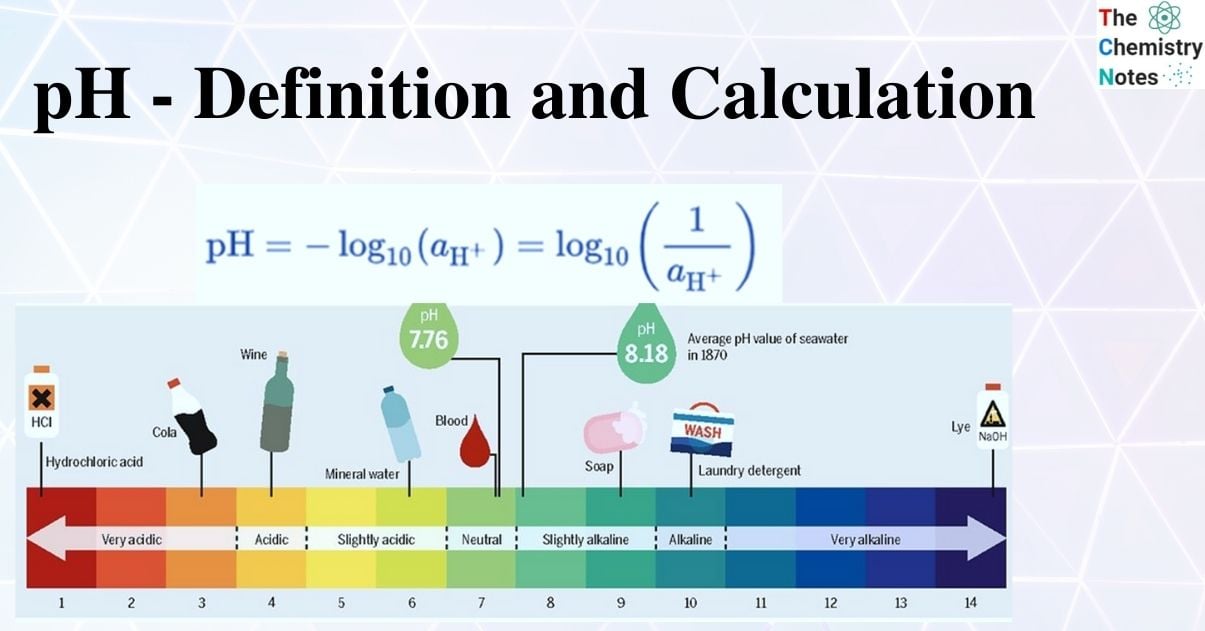
The pH level is determined by the activity of hydrogen atoms, which is a good indicator of the acidity or alkalinity of water. The scale ranges from 0 to 14, with 7.0 being neutral. Water with a low pH is said to be acidic, while water with a high pH is said to be basic, or alkaline.
Acids and Bases Review
There are several ways to define acids and bases, but pH specifically refers to the concentration of hydrogen ions in aqueous (water-based) solutions. Water dissociates into a hydrogen ion and a hydroxide.
Water shows amphoteric nature (it can act as both an acid and a base). Two water molecules react to produce hydronium and hydroxide ions:
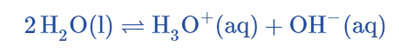
This is also called the self-ionization of water.
This equation also can be represented as;
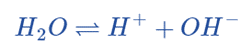
The reaction can be shifted to the reactants or products, as expected for any equilibrium:
- When an acid (H+) is added to water, the equilibrium shifts to the left, and the concentration of OH– ions decreases.
- When a base (OH–) is added to water, the equilibrium shifts to the left, and the concentration of H+ decreases.
When calculating pH, keep in mind that [ ] stands for molarity, M. Molarity is measured in moles of solute per liter of solution. If the concentration is given in a unit other than moles (mass percent, molality, etc.), convert it to molarity before applying the pH formula.
The relationship between pH and molarity can be expressed as:
Kw = [H+] [OH–] = 1 x 10-14 at 25°C
for pure water [H+] = [OH–] = 1 x 10-7
- Kw is the dissociation constant of water
- Acidic Solution: [H+] > 1×10-7
- Basic Solution: [H+] < 1×10-7
The pH of Acids and Bases
A solution’s pH ranges from 0 to 14.
Acidic solutions have a value ranging from 0 to 7 on the pH scale.
Basic solutions have a value ranging from 7 to 14 on the pH scale.
Neutral solutions are those that have a value of 7 on the pH scale.

Solutions with a pH value of 0 are known to be strongly acidic. Furthermore, the acidity decreases as the value increase from 0 to 7, whereas solutions with a pH equal to 14 are classified as strongly basic. As the value decreases from 14 to 7, the basicity decreases. The number of H+ and OH– ions produced determines the strength of acids and bases. Acids with higher H+ ions are considered strong acids, and vice versa.
The degree of ionization of acids and bases varies depending on the acid and base. It aids in determining the strength of acids and bases. The concentration of hydronium ion (H3O+) also influences acid strength. We can tell the difference between acids and bases by comparing the concentrations of hydronium ions and hydroxyl ions.
Acidic solution: [H3O+] > [OH–]
Neutral solution: [H3O+] = [OH–]
Basic solution: [H3O+] < [OH–]
Difference Between Acid and Base
pH calculations
You can calculate the pH of a chemical solution, or how acidic or basic it is. Anything less than 7 is acidic, and anything greater than 7 is basic. Check out the steps below to learn how to find the pH of any chemical solution. To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity).
The equilibrium equation yields the following formula for pH:
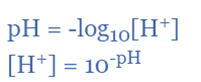
In other words, pH equals the negative log of the molar hydrogen ion concentration or the molar hydrogen ion concentration multiplied by 10 to the power of the negative pH value. This calculation is simple to perform on any scientific calculator because most of them have a “log” button. It differs from the “ln” button, which stands for the natural logarithm.
Calculating pH values from [H+]
Calculate the pH of a solution whose H+ ion concentration is 5.32 × 10–4 mol dm–3.
pH = – log10 [H+]
= – log10 (5.32 × 10–4)
= 3.27
Calculating [H+] from pH
Calculate the hydrogen ion concentration of a solution whose pH is 10.5.
pH = –log10 [H+]
[H+] = 10–pH
= 10–10.5
= 3.16 × 10–11 mol dm–3
The pH of strong acids
Each molecule of monobasic acid contains only one replaceable hydrogen atom. In solution, strong monobasic acids like hydrochloric acid are completely ionized. As a result, the concentration of hydrogen ions in the solution is approximately equal to the concentration of acid. (We assume that the concentration of H+ ions produced by the ionization of water molecules is very low in comparison to those produced by acid.)
Diluting the acid ten times reduces the H+ ion concentration by one-tenth while increasing the pH by one.
Calculating the pH of strong bases
Strong bases, like sodium hydroxide, completely ionize in solution. The concentration of hydroxide ions in a sodium hydroxide solution is thus approximately the same as the concentration of sodium hydroxide.
To calculate the pH of a solution of a strong base we need to know:
- concentration of OH– ions in the solution
- equilibrium expression for the ionization of water: Kw = [H+] [OH–]
- value of Kw for water.
Since, Kw = [H+] [OH–]
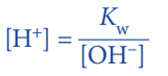
We can calculate the [H+] and then calculate the pH.
The significance of pH
- A living organism can only tolerate a narrow range of pH changes; any further change in pH makes survival difficult. In the case of acid rain, for example, the pH of the water is less than 7. It lowers the pH of river water as it flows into it, making aquatic life survival difficult.
- We all know that our stomachs contain hydrochloric acid, which aids in food digestion. We experience a lot of pain and irritation when our stomach produces too much hydrochloric acid during indigestion. As a result, we typically use antacids or a mild base to increase the pH of the acidic stomach and thus reduce pain.
- Bacteria in our mouth can lower the pH of our mouth by producing acids through the degradation of food particles. As a result, we are told to brush our teeth with toothpaste (which is generally basic) to prevent decay by maintaining the pH.
- We feel a lot of pain when stung by a bee because the bee injects methanoic acid through its sting. As a result, we are generally advised to apply baking soda or other mild bases to the surface to help maintain the pH.
Do watch the video for additional information about pH.
References
- Bates, Roger G. Determination of pH: theory and practice. Wiley, 1973
- Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel’s Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, ISBN 0-582-22628-7, Section 13.23, “Determination of pH”
- https://byjus.com/chemistry/ph-of-acids-and-bases/
- https://www.thoughtco.com/how-to-calculate-ph-quick-review-606089
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acids_and_Bases_in_Aqueous_Solutions/The_pH_Scale
- https://www.albert.io/blog/how-to-calculate-ph-in-chemistry/
- https://www.britannica.com/science/pH

