Paper chromatography is a simple yet effective detective tool for separating and analyzing the constituents of a mixture. Because paper chromatography is so similar to thin-layer chromatography (TLC), it is often used to teach TLC and other chromatography techniques. It is an inexpensive method for separating dissolved chemical substances based on the different rates at which they move across sheets of paper. It is a resource-efficient analytical tool. Partition chromatography is demonstrated by paper chromatography.
Paper chromatography was developed in 1943 by Synge and Martin.
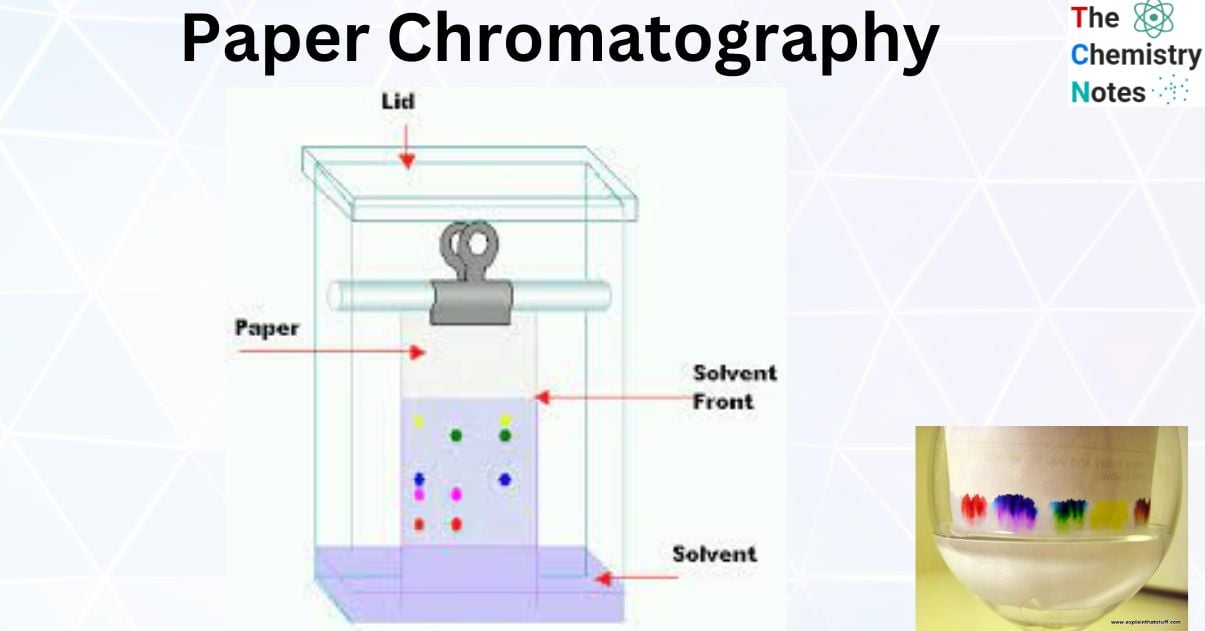
What is Paper Chromatography?
Paper chromatography is a chromatography technique in which a solution is forced to flow through paper sheets or strips as the adsorbent and stationary phase.
In analytical chemistry, paper chromatography is a technique used to separate dissolved chemical substances by taking advantage of their different rates of migration across paper sheets.
In this procedure, chromatography paper serves as the stationary phase and is suspended in a mixture of solvents, which also serves as the mobile phase. The mixture to be separated is placed in a region at the bottom of the chromatographic paper, and as the solvent moves up the paper, the components are carried to varying degrees depending on how well they adhere to the paper. The components are consequently separated at various heights.
Principle of Paper Chromatography
Paper chromatography is a form of “partition” chromatography, in which the mobile phase is primarily a liquid or gas and the stationary phase is a liquid or a liquid supported on an inert solid. According to the principle of differential portioning between the mobile and stationary phases, various molecules are separated.
- In this method of chromatography, a support made of highly purified cellulose filter paper is used, and a stationary “liquid phase” is created when water drops condense in the pores of the support.
- The mobile phase is the fluid that is put into a developing tank or jar. It can also be referred to as “liquid-liquid” chromatography because both phases are in a liquid state.
- If a mixture of products in a solvent exhibits significantly different portioning or different partition coefficients between the mobile phase and the stationary phase, such as water-saturated cellulose, the mixture can be separated based on its constituents.
- When the aqueous phase is given preference in the partition, the solute will typically stay close to the application site. In contrast, when the mobile organic phase is given preference, the solute will move with the solvent flow.
Stationary Phase and Mobile Phase
Stationary Phase: It is liquid—that is, water that has been entrapped in the molecular structure of the paper. The matrix of cellulose fibers in chromatography paper serves as a supporting material for the stationary phases. There are three running characteristics for chromatography papers: slow, medium, and fast.
Whatman No. 1 or a comparable paper is most frequently used for chromatographic analysis. In general, the paper has 98–99% α–cellulose as well as 0.3–1% β–cellulose content.
Mobile Phase: In a pure paper system, the eluent is typically a liquid, such as a single solvent or a combination of solvents that can pass through the paper.
In mixed solvent systems:
Isopropanol: ammonia:water 9:1:2
Methanol : water 4:1
N-butanol : glacial acetic acid : water 4:1:5
The series of solvents called “eluotropic” is a group of solvents arranged in increasing order of polarity, and these are the solvents that were used.
A solute’s mobility in a solvent is typically inversely correlated with how soluble it is in that solvent. A solute will travel with the solvent because it dissolves more easily in the mobile phase, causing a partition between the two phases.
Types of Paper Chromatography
Ascending Paper Chromatography
The solvent is found to be developing and moving upward. In this instance, enough mobile phase is added to the development chamber. The sample and reference are marked on a line drawn a few centimetres from the bottom edge of the paper, which is then suspended from a hook at the top.
Descending Paper Chromatography
The solvent front moves down the length of paper suspended from the chamber’s top. The upper chamber contains a trough where the mobile phase is stored. The paper is tightened to the top with spotting on the line drawn a few centimetres from the top. The jar is covered and filled with the mobile phase vapor to achieve equilibrium before elution.
Ascending – Descending Chromatography
The solvent initially moves upward on the folded paper over the rod before turning around and moving downward in this particular type of chromatography.
Circular or horizontal
By moving the liquid phase radially, this enables the separation of sample components into concentric circular zones.
Paper Chromatography Procedure
The following describes the fundamental steps involved in performing paper chromatography:
- Choosing an appropriate type of development
Based on factors like the complexity of the solvent, the type of paper being used, the nature of the sample, etc., the type of development is chosen. Radial chromatography is frequently chosen because of its simplicity of use and high resolution. Additionally, it takes less time to complete and produces repeatable results. - Choosing the right filter paper
The best type of filter paper to use can be determined by the sample quality and pore size of the filter paper. Whatman No. 1 filter paper, which is typically used, is typically used as a thin layer. - Sample Preparation
The sample is prepared by dissolving it in an appropriate solvent. It should be inert with the sample being analyzed, and the mobile phase is typically used for this. - Mark the sample on the paper
The sample should be properly positioned on the paper and should be properly spotted using a capillary tube in the center. - Chromatogram development
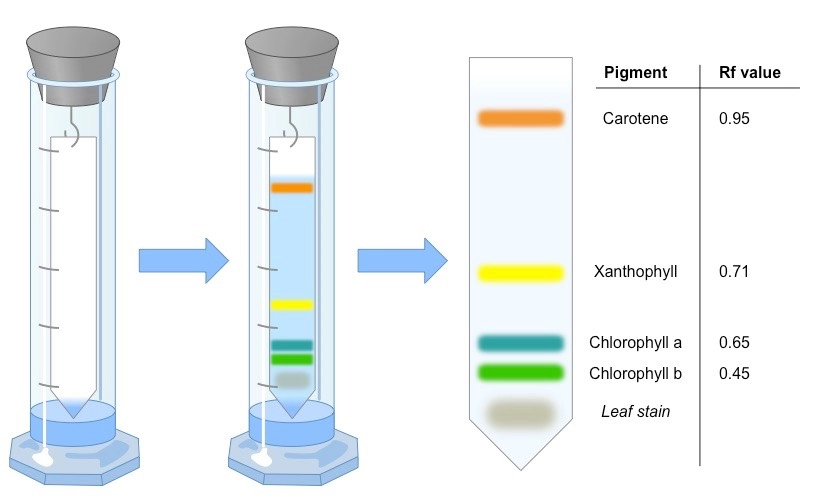
The mobile phase is used to develop the chromatogram on paper. When the mobile phase is attracted to the filter paper by capillary action, the components of the samples begin to move in accordance with their affinity for the mobile phase. - Compound detection and paper drying
After the chromatogram has developed, the paper is first allowed to air dry at room temperature. Thereafter components are recognized through the use of detecting agents and are indicative of various chemical compounds.
Applications of Paper Chromatography
There are many different fields where paper chromatography is applicable.
- This technique is helpful for isolating and purifying mixtures’ individual components.
- Analysis of food colors in artificial drinks, ice cream, sweets, and other foods.
- With the help of forensics, drugs and their metabolites can be identified and put up for comparison with reference standards. The analysis of samples that are available in milligram or microlitre quantities is also made possible by paper chromatography.
- Information about new drug molecule development, reaction completion, and manufacturing process advancement is provided by the pharmaceuticals industry. Because this process is inexpensive, it is used as an alternative method of monitoring the active ingredients present in drug forms.
Advantages and Disadvantages of Paper Chromatography
Advantages
- This technique necessitates a minimal amount of quantitative material.
- It is less expensive than other chromatography methods.
- This method can identify both unknown inorganic and organic compounds.
- It takes up little space when compared to other analytical methods or equipment.
Disadvantages
- It cannot handle large amounts of sample.
- It is ineffective in quantitative analysis.
- Paper chromatography cannot however separate complex mixtures.
- Less Accurate than HPLC or HPTLC
Reference
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/paper-chromatography/
- To perform paper chromatography, https://doi.org/10.1016/B978-0-12-824449-4.00029-3
- https://lab-training.com/paper-chromatography/
- https://chemdictionary.org/paper-chromatography/
- https://chem.libretexts.org/Ancillary_Materials/Laboratory_Experiments/Wet_Lab_Experiments/General_Chemistry_Labs/Online_Chemistry_Lab_Manual/Chem_11_Experiments/03%3A_Paper_ChromatographySeparation_and_Identification_of_Five_Metal_Cations(Experiment)
- https://microbenotes.com/paper-chromatography/
