The Chemistry Notes is an educational niche website related to chemistry (Basic Chemistry, Physical Chemistry, Organic Chemistry, Inorganic Chemistry, Biochemistry, Periodic Table, etc.) and different other branches of chemistry to provide chemistry notes for high school, undergraduate, and graduate students.
Notes Categories
Latest Notes
- Potassium Dichromate: Preparation, Properties, Reactions, Uses, Health Effects

- Bond Cleavage: Homolysis and Heterolysis

- Salt: Preparation, Properties, Types, Uses

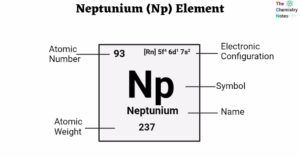
- Neptunium (Np) Element: History, Properties, Uses, Hazards
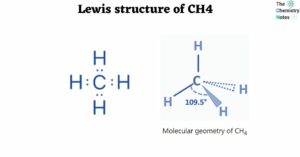
- Lewis Structure of CH4
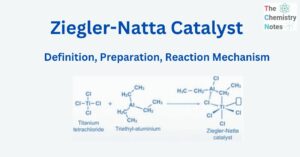
- Ziegler-Natta Catalyst: Definition, Preparation, Reaction Mechanism
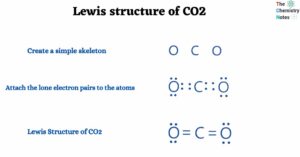
- Lewis structure of CO2
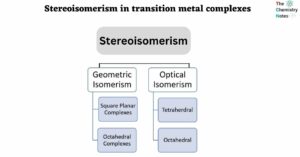
- Stereoisomerism in transition metal complexes
- Acidic Strength of Carboxylic Acids

- Halogenation of Alkene: Mechanism, Stereochemistry