Ozone is a condensed form of oxygen with the molecular formula O3. German chemist C.F. Schonbein (1799–1868) was the first to recognize ozone in 1840, he recognized that the smell produced while sparking was caused by an unidentified substance that he named ozone. Twenty years later, the new substance was discovered to be a triatomic allotrope of oxygen: in 1856, Thomas Andrews demonstrated that ozone was exclusively formed by oxygen. In 1863, Soret established the relationship between oxygen and ozone by discovering that three volumes of oxygen result in two volumes of ozone. Ozone is a triatomic allotrope of oxygen (an oxygen molecule with three atoms instead of two as in the usual form
It is responsible for the recognizable odor of the air during a thunderstorm or in immediate contact with electrical equipment. It is present in small amounts in the sea air while in large quantities in the atmosphere at altitudes of 12 to 15 miles above the earth’s surface. In specific circumstances, photochemical reactions in the earth’s atmosphere between nitrogen oxides and hydrocarbons can form ozone in concentrations greater enough to irritate mucous membranes and the eyes.
Interesting Science Videos
Laboratory preparation of ozone
In the laboratory, It is prepared by passing the electrical discharges through dry oxygen in an apparatus known as an ozoniser. The commonly known ozonisers are:
- Siemen’s ozoniser
- Brodies’s ozonizer
1. Siemen’s ozoniser
It consists of two metal tubes that are joined at one end and sealed. Tin foil is used to cover the outer and inner tubes, which are each attached to one terminal of an induction coil. A low-temperature current of pure, dry oxygen is passed across the annular space between two tubes, subjecting the gas to a series of silent electric discharges.
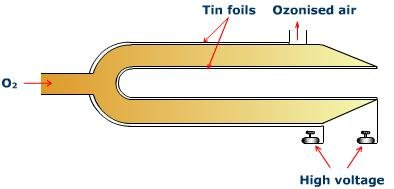
Source: https://www.toppr.com/ask/en-np/content/concept/ozoniser-203532/
Ozone is formed by the partial condensation of oxygen. The gas leaving the tube is a mixture of O2 and O3.
3 O2 → 2 O3
Conversion of ozonised O2 into pure ozone: The ozonized oxygen is cooled in liquid air. At -112.4 o C, ozone condenses, whereas oxygen can become liquid at -182.5 o C. It is possible to obtain pure, oxygen-free ozone by letting the liquid evaporate.
2. Brodie’s Ozoniser
The equipment is made up of a U-shaped glass tube. The ozonizer is immersed in a cylinder of diluted H2SO4 that dips one platinum electrode. The other platinum electrode is also immersed in the diluted H2SO4 in the inner tube. Pure dry oxygen is passed through the annular space and is converted into ozonized oxygen under the influence of silent electric discharge when the two platinum electrodes are connected to the terminals of a working induction coil.
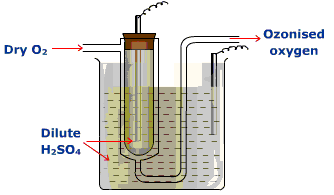
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_
The_Oxygen_Family_(The_Chalcogens)/Z008_Chemistry_of_Oxygen_(Z8)/Ozone
Structure
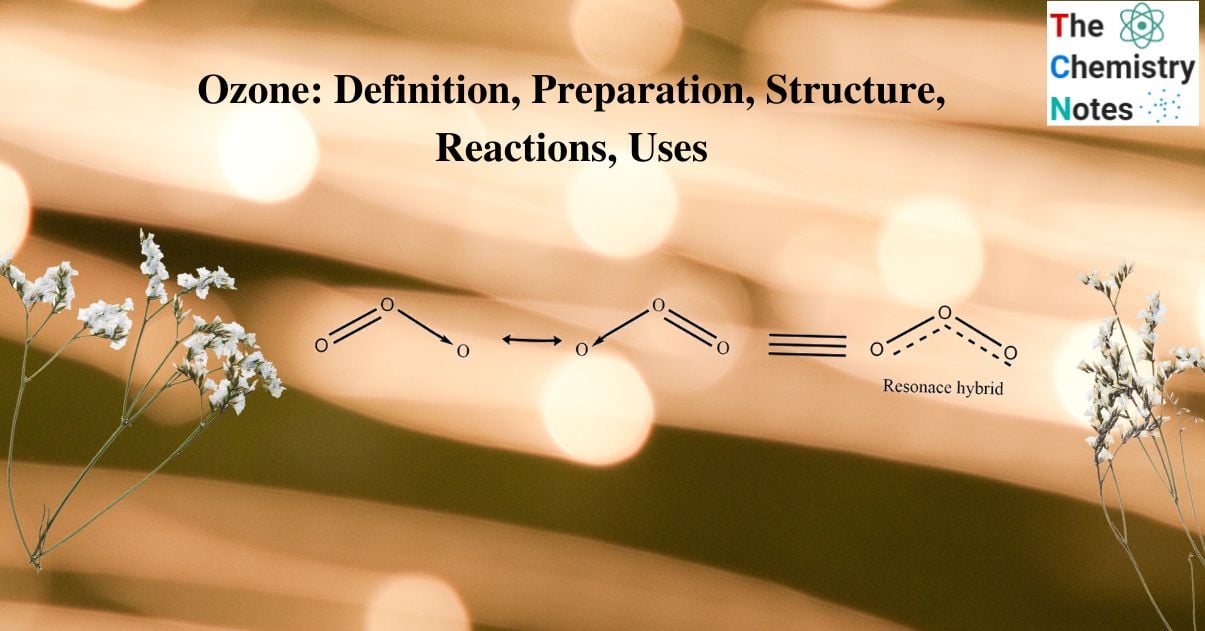
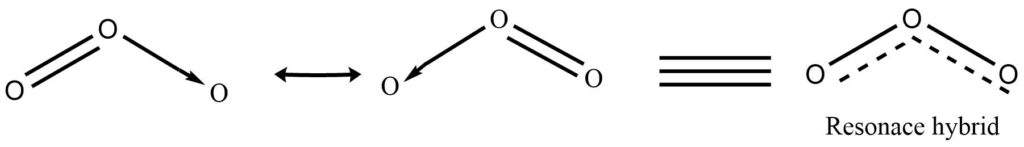
It has an angular geometry with a bond length of 1.278 Ao and a bond angle of 1160 48′. It is a V- shaped molecule. Microwave spectroscopy indicates that it has similar symmetry to that of the water molecule. It has the same isoelectronic characteristics as the nitrite anion. The structure is best described as the resonance hybrid.
Physical properties
- It is pale-blue gas.
- It has bad fishy smell.
- It is slightly soluble in water and appreciable soluble in turpentine oil.
- At -112.4 °C, it condenses to produce a dull blue fluid. It hardens into crystals of dark violet after further cooling.
- It has has a weak paramagnetic property.
Chemical properties
Decompostion
When heated, it tends to break down into oxygen. At 300 °C the decomposition is completed in the presence of a catalyst black and manganese dioxide.
3 O3 → 2 O2
Oxidising property
It serves as a strong oxidizing agent. As it breaks down, it creates nascent oxygen, making it a much stronger oxidizing agent than dioxygen.
O3 → O2 + O
I. Oxidation of metals
It directly combines with metals like Ag, Hg and Cu to form respective oxides.
2 Ag + O3 → Ag2O + O2
2 Cu + O3 → Cu2O + O2
II. Oxidation of metalloids
Metalloids, like arsenic and antimony, in reaction with ozone, produces respective oxy-acids.
Antimony in reaction with ozone oxidized into antimonic acid.
2 Sb + 5 O3 + 3 H2O → 2 H3SbO4 + 5 O2
Arsenic in reaction with ozone oxidized into arsenic acid.
2 As + 5 O3 + 3 H2O → 2 H3AsO4 + 5 O2
III. Oxidation of non metals
In presence of water, non metals like iodine, sulphur, etc reacts with ozone to produce respective oxy acids.
a. Iodine is oxidised into iodic acid.
I2+ 5 O3 + H2O → 2 HIO3 + 5 O2
b. Sulphur on reaction with ozone produces sulphuric acid.
S+ 5 O3 + H2O → 2 H2SO4 + 3 O2
c. On reaction with ozone, phosphorous is oxidised into Phosphoric acid.
P+ 5 O3 + 3 H2O → 2 H3PO4 + 5 O2
IV. Oxidation of compounds
i. On reaction with ozone, lead sulphide is oxidised into lead sulphate.
PbS + 4 O3 → 2 PbSo4 + 4 O2
ii. Iodides reacts with O3 to produce iodine as oxidised product.
2 KI + H2O + O3 → 2 KOH + I2 + O2
iii. Nitrites undergoes oxididation reaction to form nitrates.
KNO2 + O3 → KNO3 + O2
iv. Potassium manganate reacts with O3 to form potassium permanganate.
2 K2MnO4 (green) +O3 + H2O → 2 KMnO4 (pink) + 2 KOH + O2
Formation of ozonoid
Alkynes and alkenes react with O3 in presence of the inert solvent at low temperatures to produce ozonides.
CH2 = CH2 + O3 → 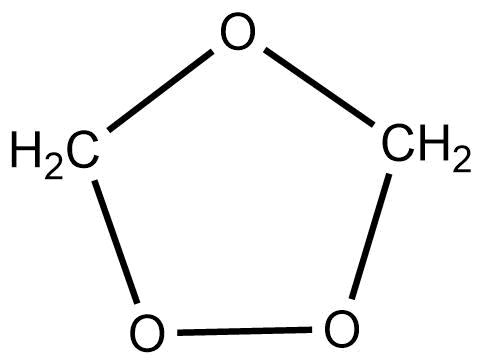
Some other reactions
In some reactions O3 molecule as whole is used without producing dioxygen.
For example, It reacts with SnCl2 / HCl or SO2 without producing dioxygen.
3 SnCl2 + 6 HCl → 3 SnCl4 + 3 H2O
3 SO2 + O3 → 3 SO3
Uses
- It is used in the sterilization of water.
- It acts as a disinfectant.
- In crowded areas like theaters, underground tunnels, and railways, ozonized air is utilized to improve the atmosphere.
- It is employed to keep meat fresh in cold storage.
- It is used to bleach delicate textiles, oil, and wax. It is used to make synthetic camphor and artificial silk.
References
- https://www.geeksforgeeks.org/ozone-properties-structure-preparation-reactions-and-uses/.
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_16%3A_The_Oxygen_Family_(The_Chalcogens)/Z008_Chemistry_of_Oxygen_(Z8)/Ozone
- https://www.toppr.com/ask/en-np/content/concept/ozoniser-203532/
- https://www.britannica.com/science/ozone.
- https://meteor.geol.iastate.edu/gccourse/chem/ozone/ozonecover_discussion.html#:~:text=Ozone%20molecules%20exposed%20to%20ultraviolet,and%20warming%20the%20upper%20atmosphere.&text=The%20free%20oxygen%20atom%20may,create%20two%20ordinary%20oxygen%20molecules.
